Introduction
|
Definition: Usual ductal hyperplasia (UDH) represents a proliferation of uncommitted epithelial cells, which reside within the epithelium of ducts and lobules. These cells possess the potential to differentiate into both luminal and myoepithelial cells, but they do not demonstrate this potential to a significant degree in usual ductal hyperplasia.
Clinical Significance: Usual ductal hyperplasia, like fibrocystic changes, typically afflicts women between the ages of thirty and sixty years. The lesion does not produce clinical or radiological signs or symptoms. Its presence may indicate a slightly elevated risk for the development of breast carcinoma; however, this possibility seems unlikely, and most physicians believe that the possible minimal increase in risk does not merit intervention. Usual ductal hyperplasia does not represent a precursor to ductal carcinoma.
Gross Findings: None
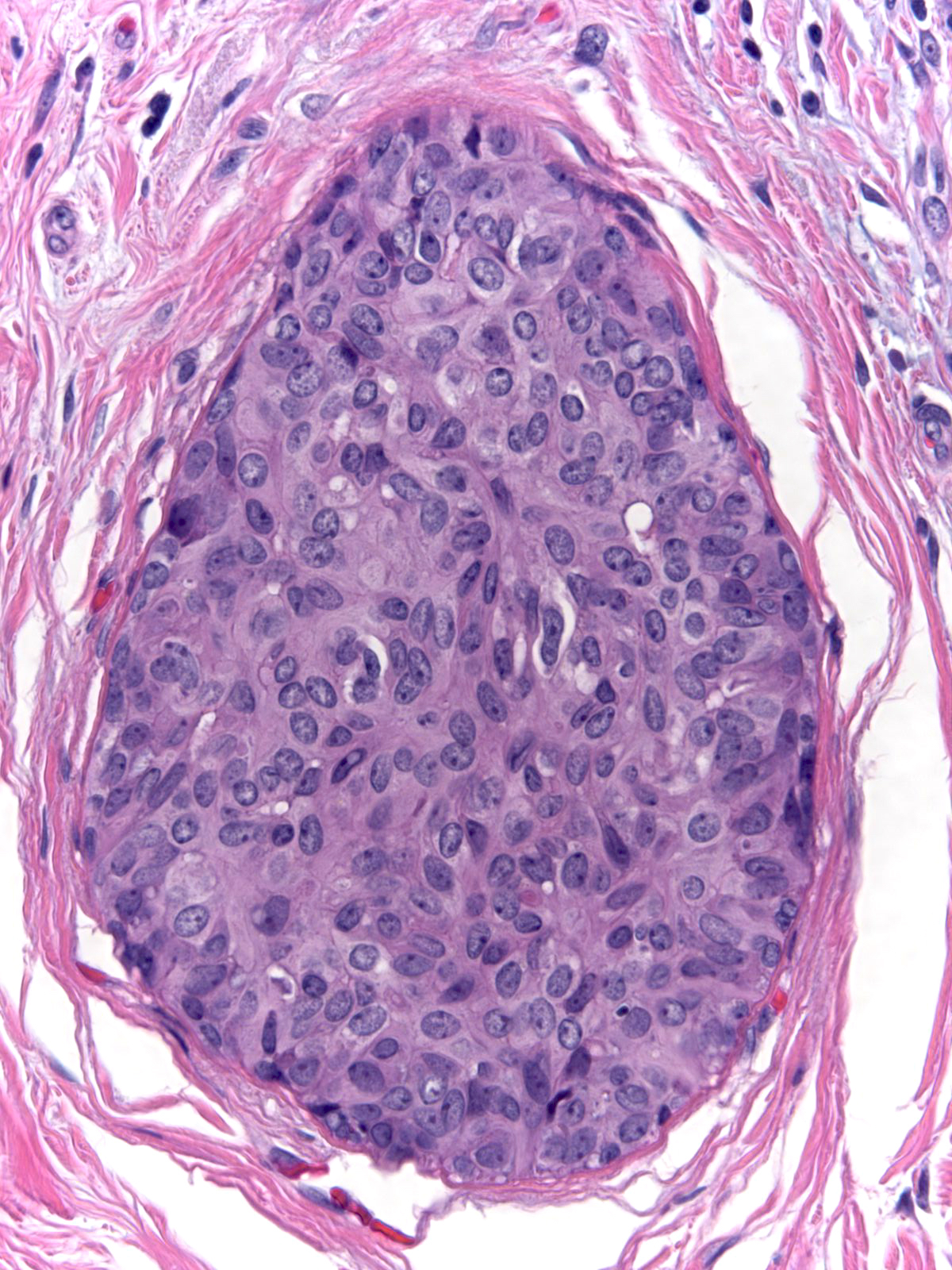
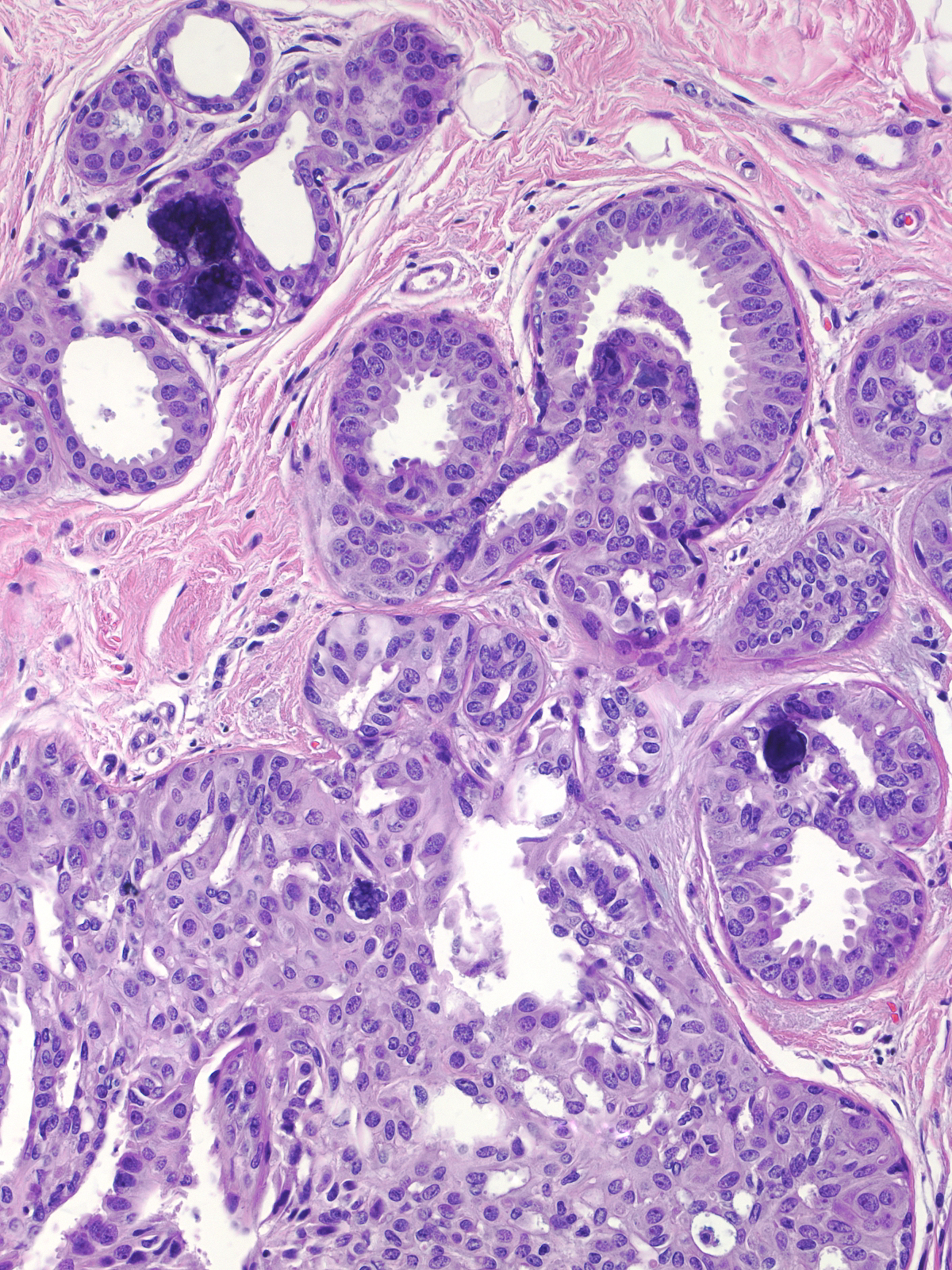
Microscopic Findings: Usual ductal hyperplasia consists of a proliferation of cohesive cells demonstrating slight cytological variability. Hyperplastic ductal cells represent the most cohesive type of proliferative mammary epithelial cells. The cells usually grow in a stratified array, but flat and micropapillary patterns occur. Stratified collections typically have spaces referred to as fenestrations within the masses. Hyperplastic cells appear irregular in their distribution, they often overlap, and they vary slightly in their nuclear characteristics.
Differential Diagnosis: Intermediate-grade ductal carcinoma in-situ (greater range in nuclear characteristics, architectural atypia, keratin 5/6 negative); flat epithelial atypia (low-grade cytologic atypia, orderly arrangement); atypical ductal hyperplasia (low‑grade cytologic atypia, polarization)
|
 UDH is a proliferation of uncommitted epithelial cells normally found in small numbers within the epithelium. UDH is a proliferation of uncommitted epithelial cells normally found in small numbers within the epithelium. |
Architectural Features of UDH
The proliferative cells usually grow into the lumen and form large collections, but flat and micropapillary growth patterns are seen, also.
 |
 |
 |
| Stratified UDH |
Flat UDH |
Micropapillary UDH |
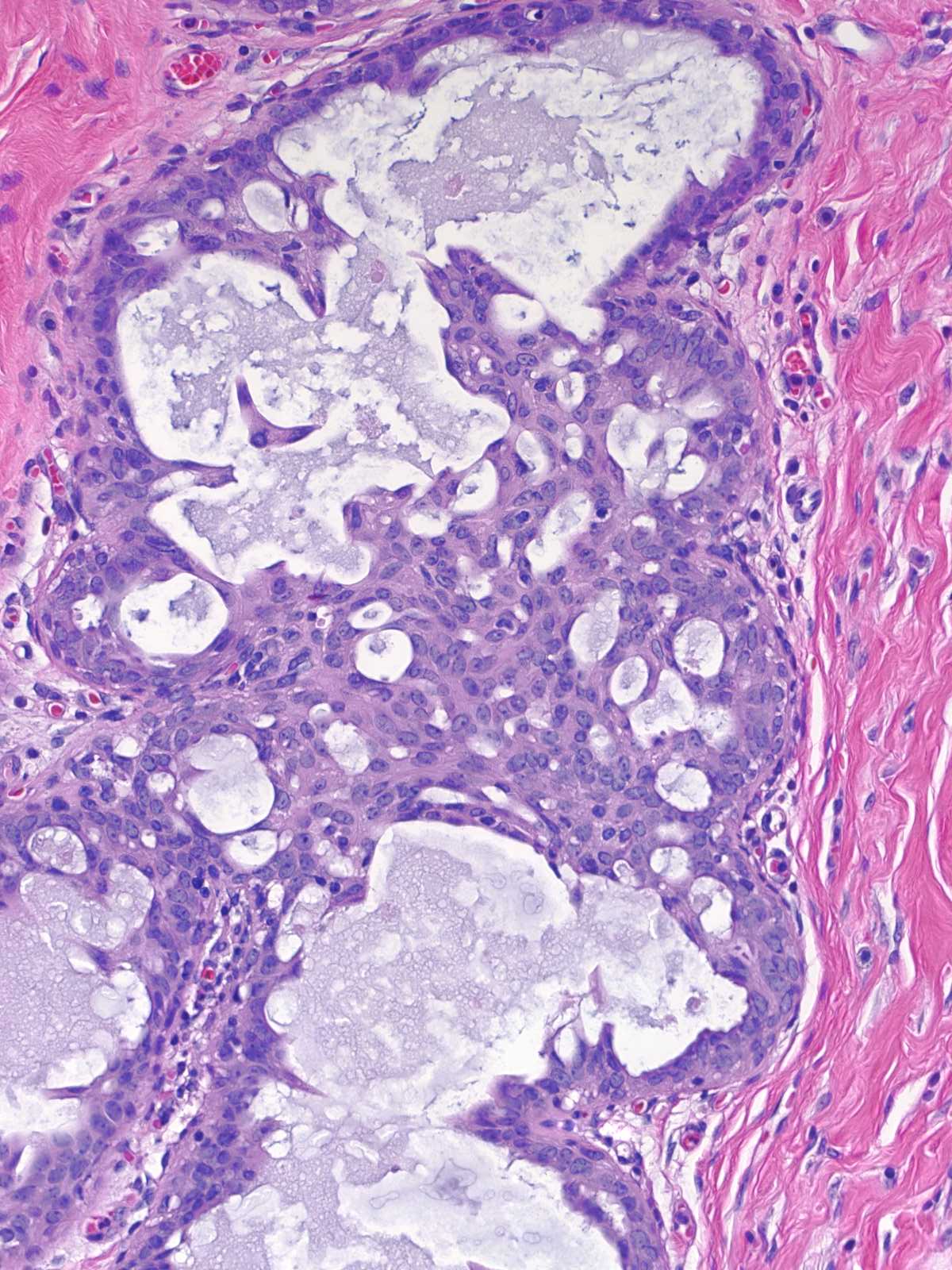
| Four architectural attributes characterize stratified examples of UDH: (1) ductular distention by central masses of cells surrounded by crescent-shaped spaces, (2) irregular fenestrations within the cell masses, (3) a streaming arrangement of the cells, and (4) maturation. |
 |
|
|
| The crescent shaped spaces encircling the central mass of cells represent remnants of the ductular lumen unoccupied by the hyperplastic cells. |
 |
|
|
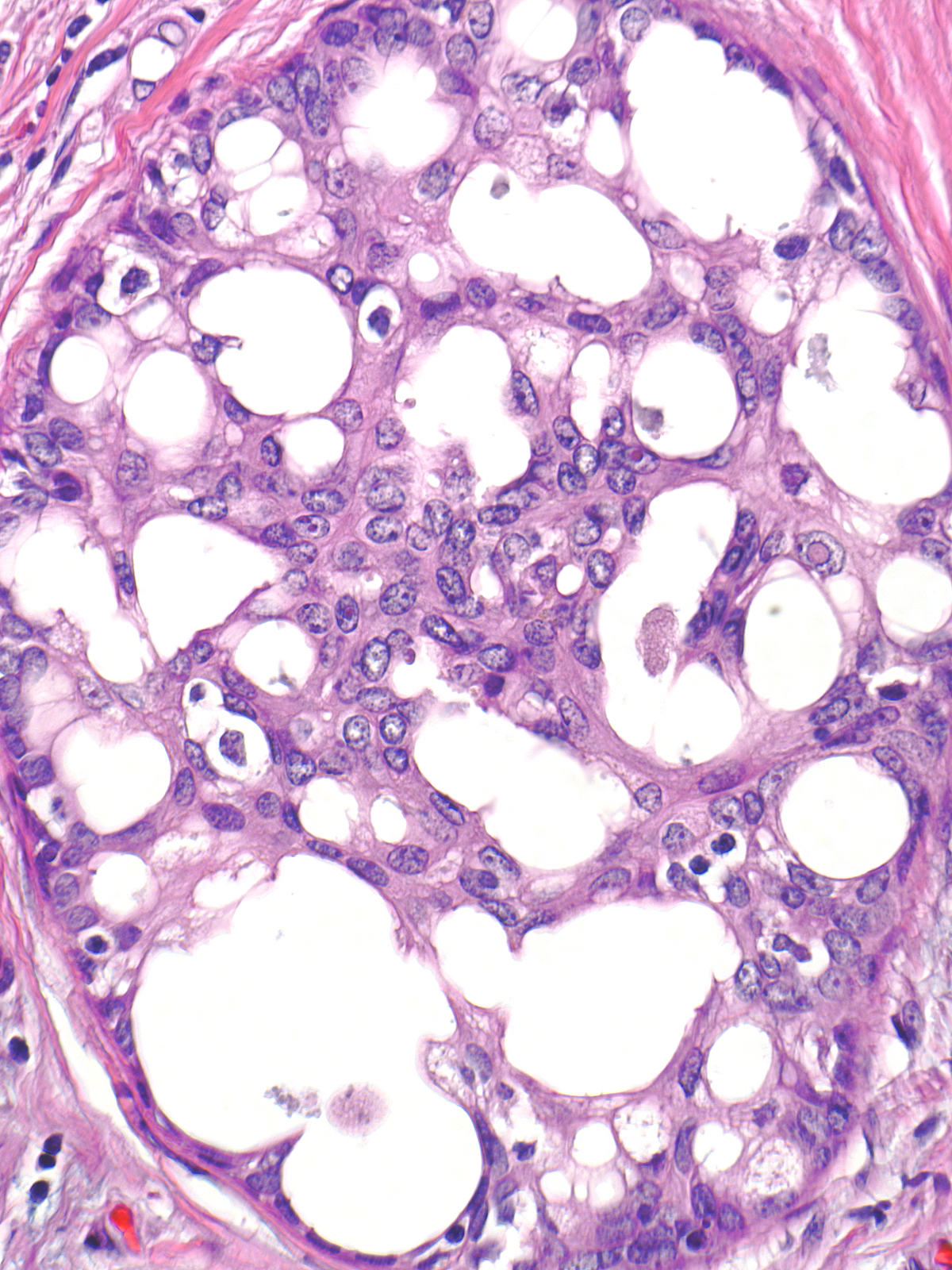
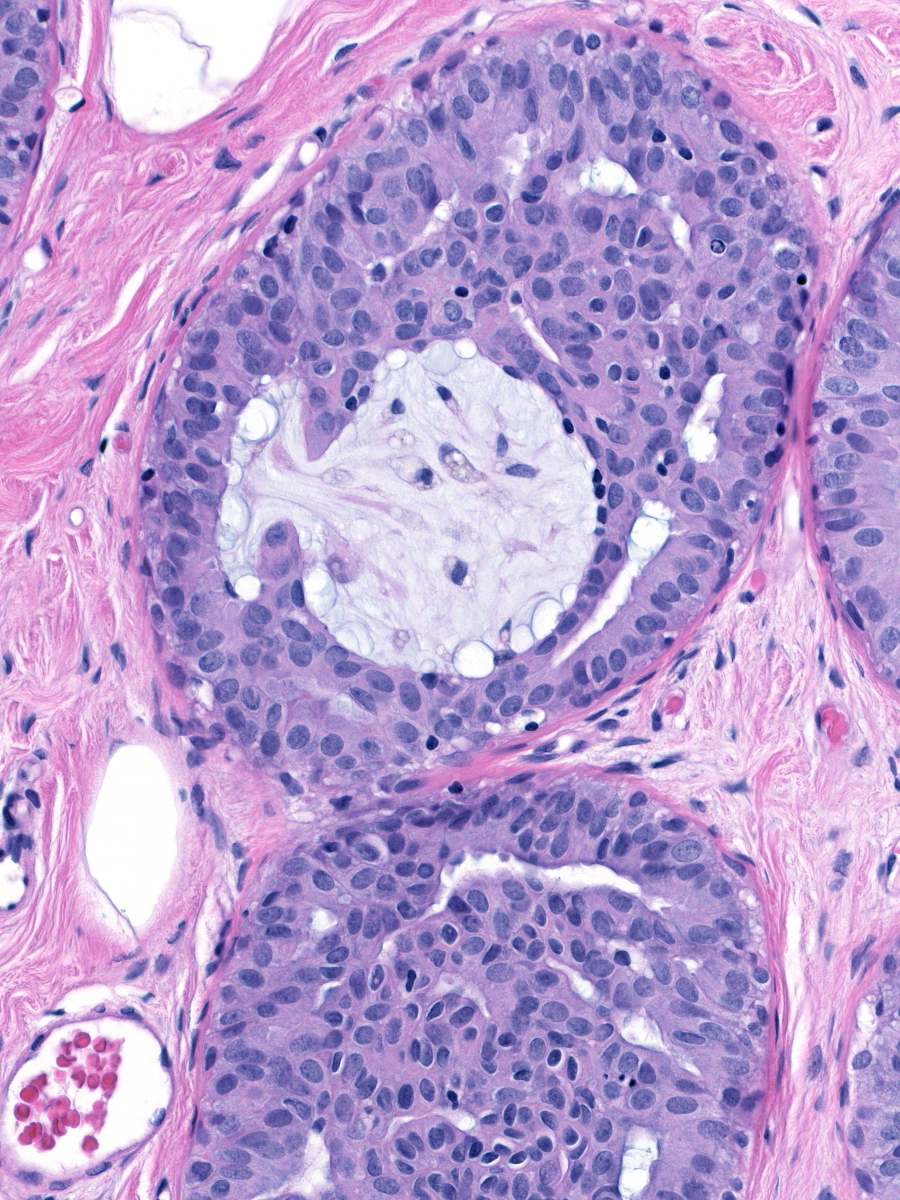
| The fenestrations within the mass, which also represent fragments of the duct lumen, have irregular, slit-like shapes. |
 |
|
|
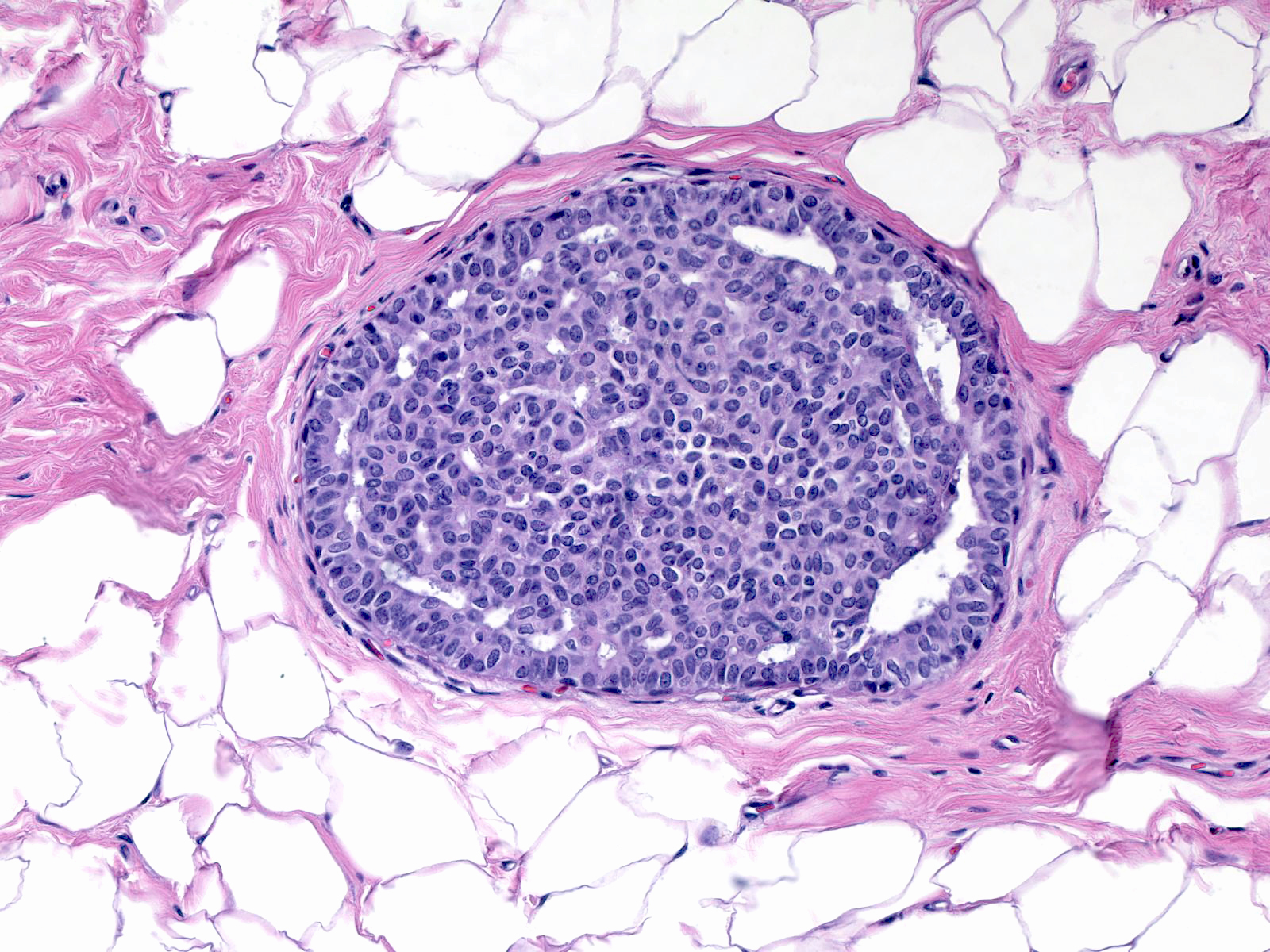
| The cells adjacent to the fenestrations do not demonstrate polarization; in fact, their nuclei often abut the luminal cell membrane. Carcinoma cells, in contrast, appear polarized and position their nuclei away from the neoplastic spaces. |
 |
|
|
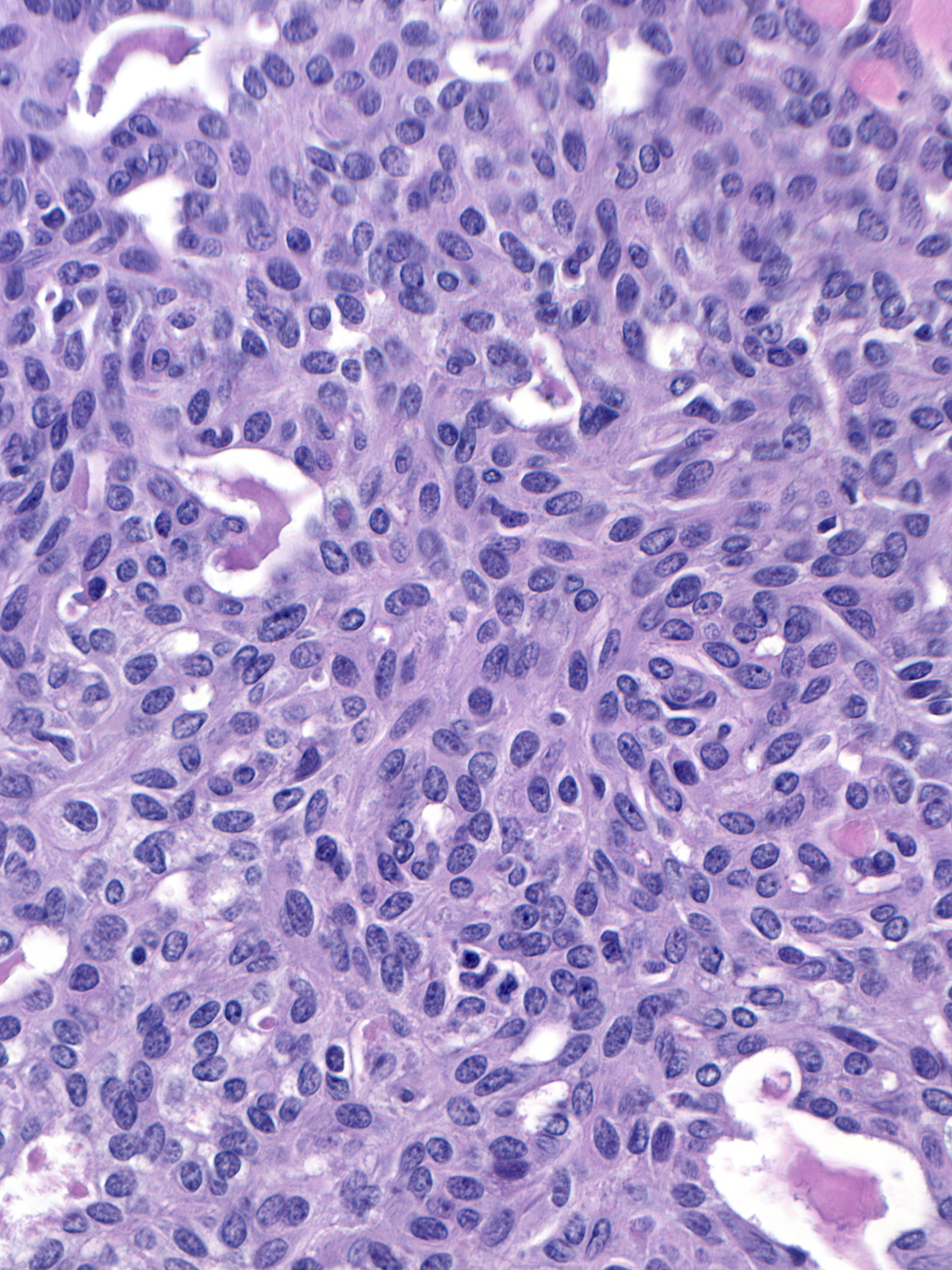
Streaming refers to an arrangement in which fusiform cells and their oval nuclei run approximately parallel to each other.
 |
 |
| Streaming |
Streaming |
In the pattern known as maturation, the cells distant from the basement membrane appear smaller than those near the basement membrane. The central (mature) cells have smaller, darker, and more closely packed nuclei and denser, eosinophilic cytoplasm compared to the larger cells near the basement membrane.
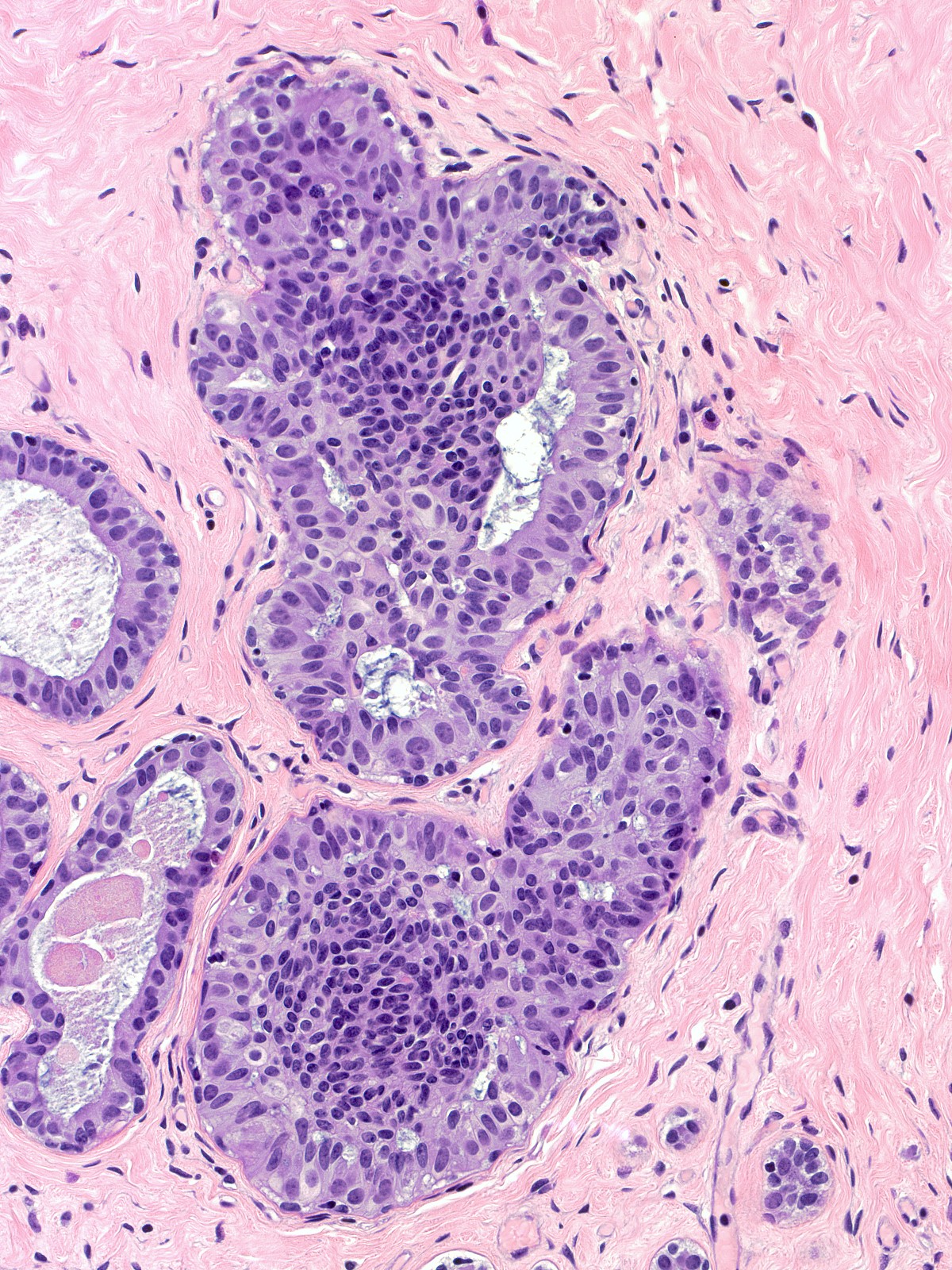 |
 |
| Maturation |
Maturation |
Bands of cells bridging the lumen can form structures known as spindle cell bridges. These structures narrow in the center of the duct, and the nuclei of the cells within the bridges run parallel to the length of the bridges. These findings give the cells a stretched appearance.
Cytological Features of UDH
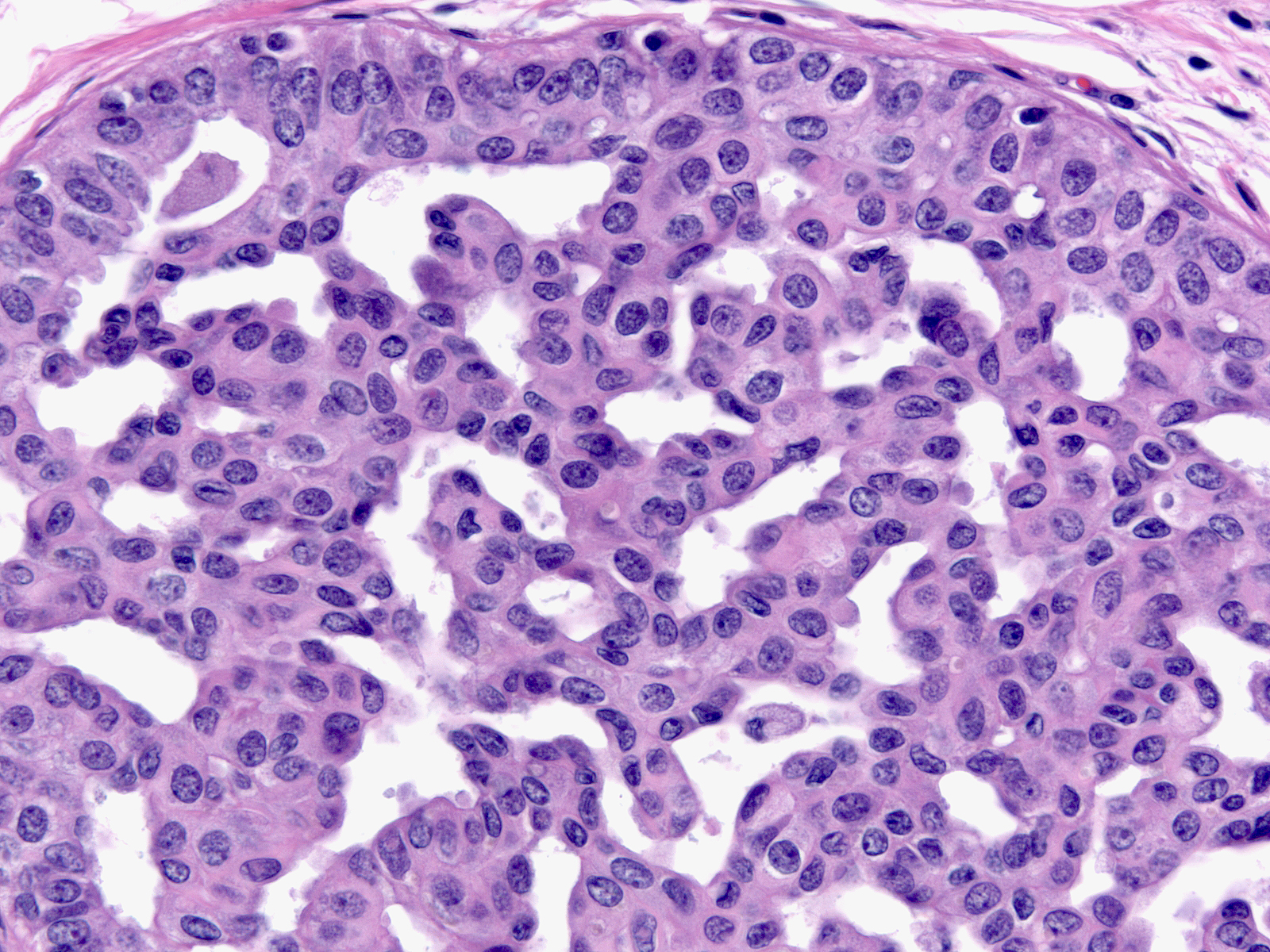
Whatever the architectural pattern, hyperplastic ductal cells display a consistent constellation of cytological features. In general, the cells appear nondescript and do not display convincing evidence of either luminal or myoepithelial differentiation.
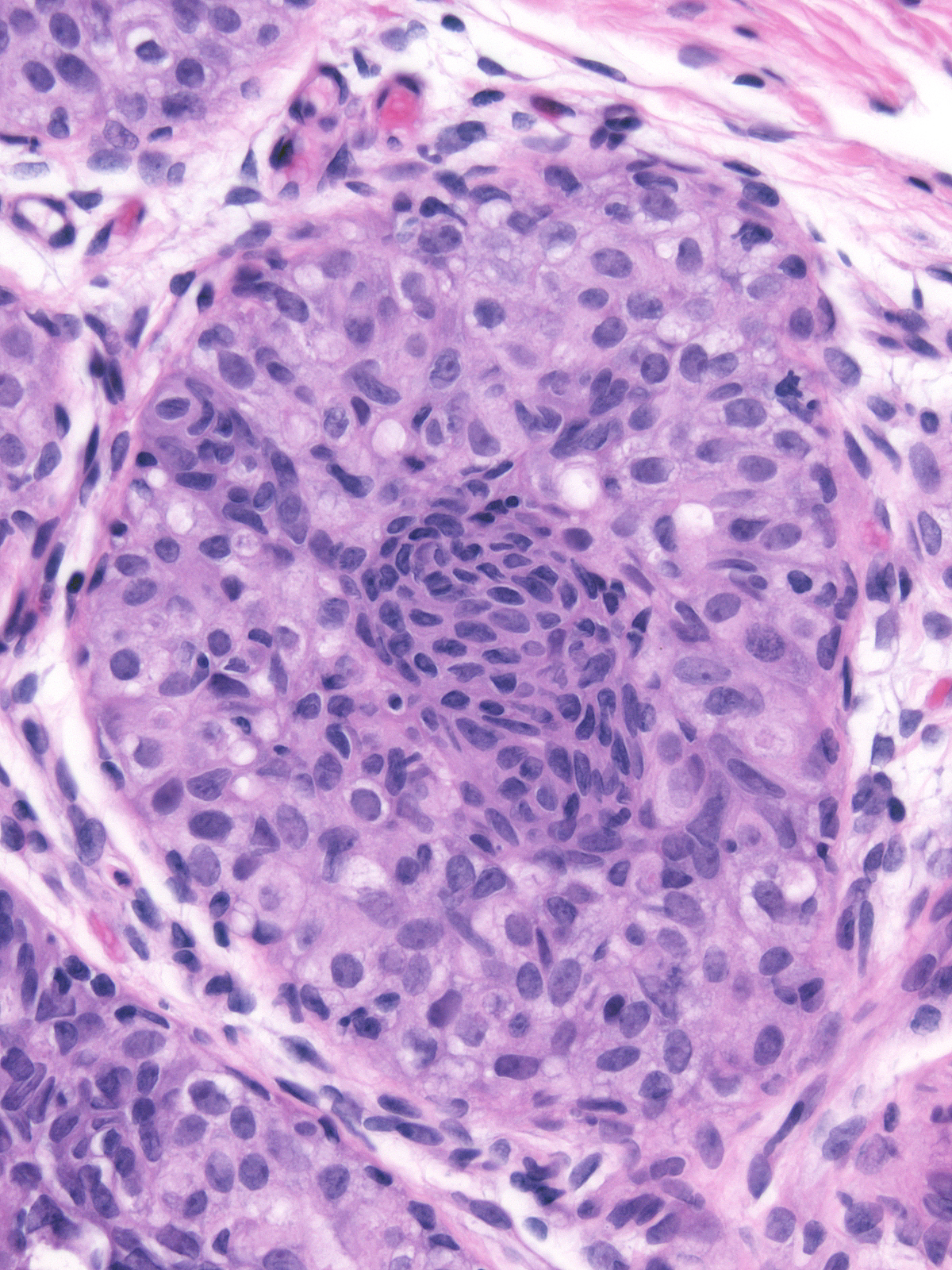
| Cell membranes do not stand out except under high magnification, so the cell masses look syncytial. |
 |
|
|
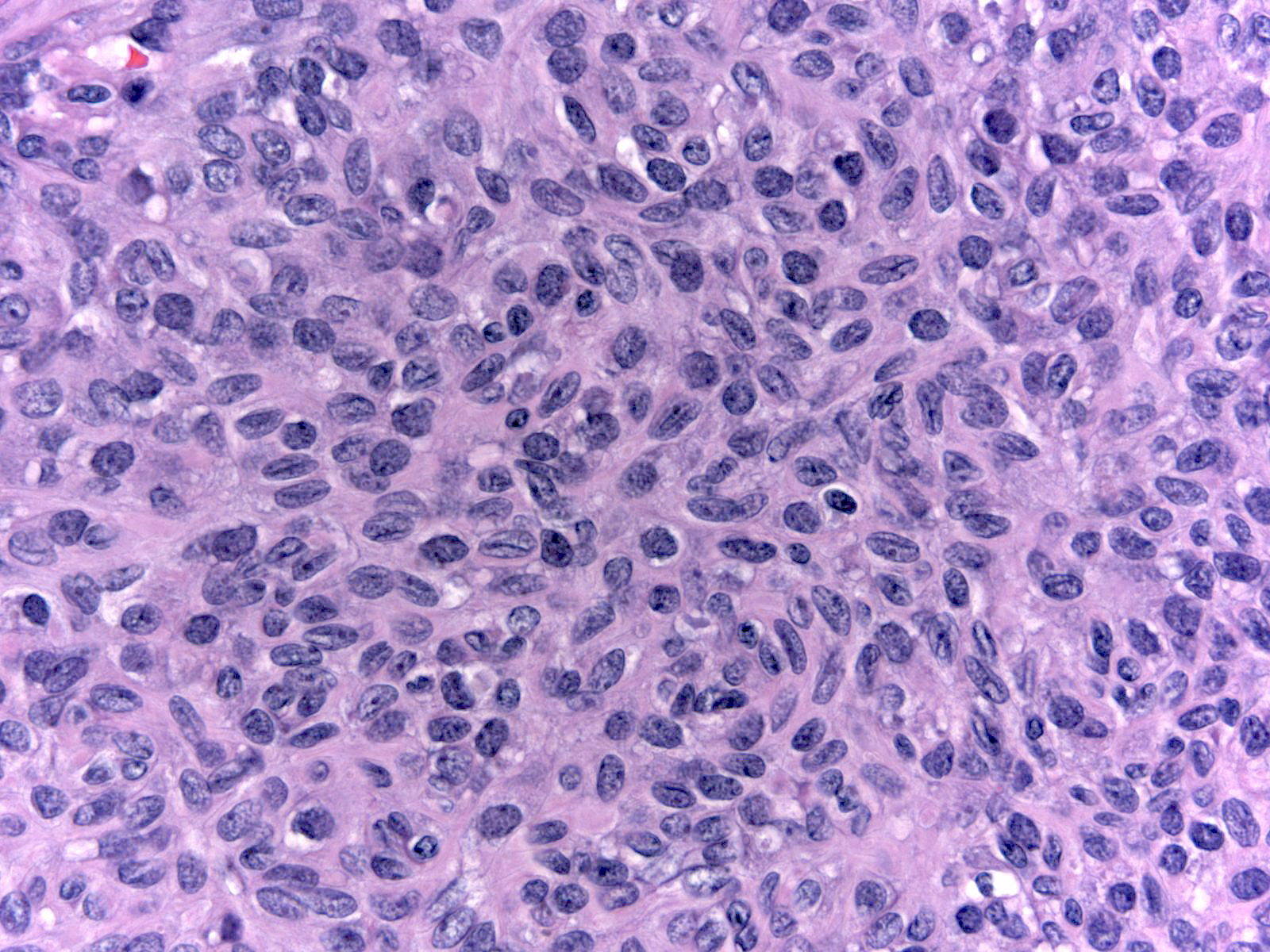
| The nuclei display an irregular distribution and they cluster and overlap. |
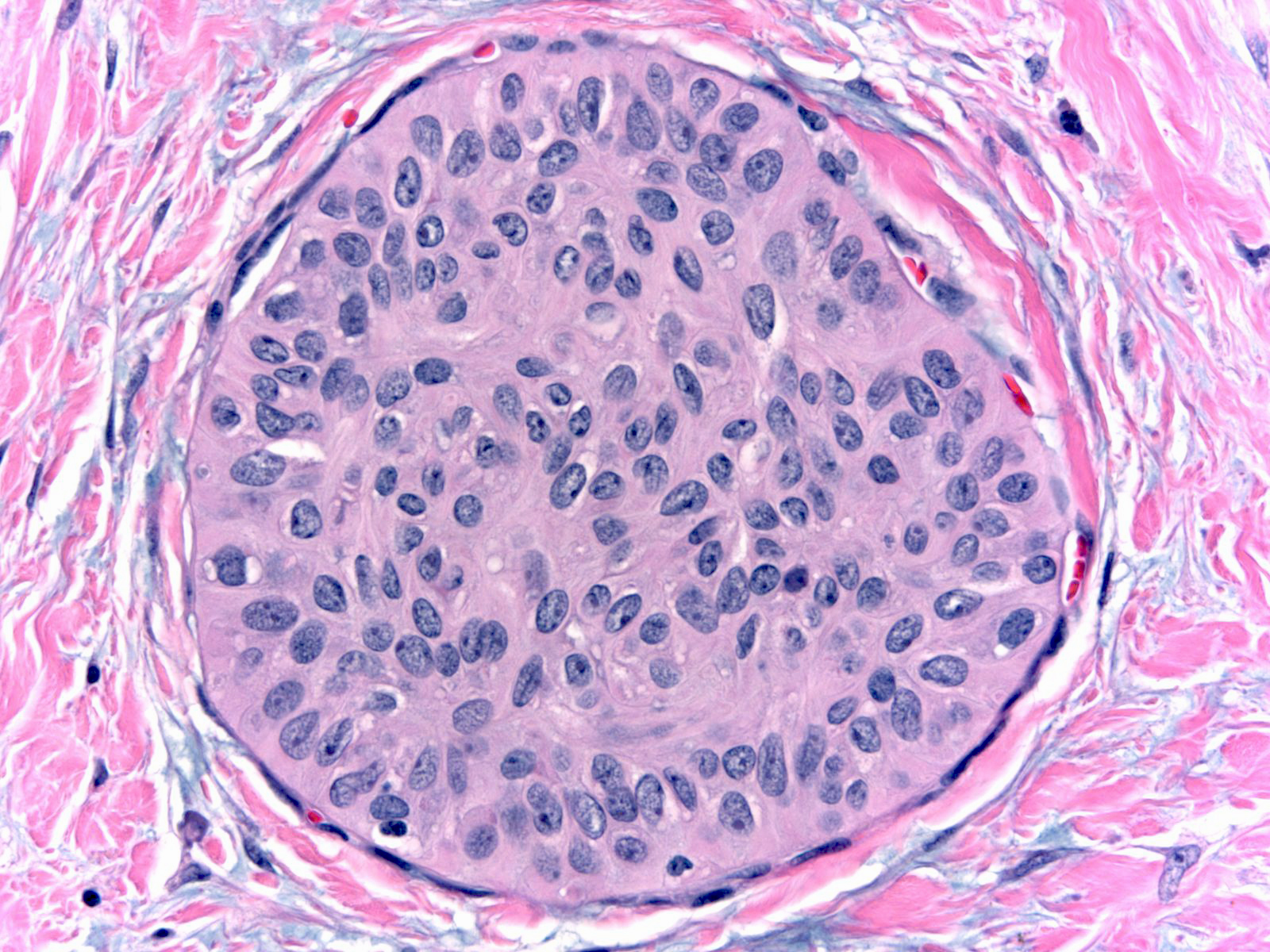 |
|
|
| The nuclei shapes vary from spindly to oval and they usually do not appear round, nor do they look uniform. |
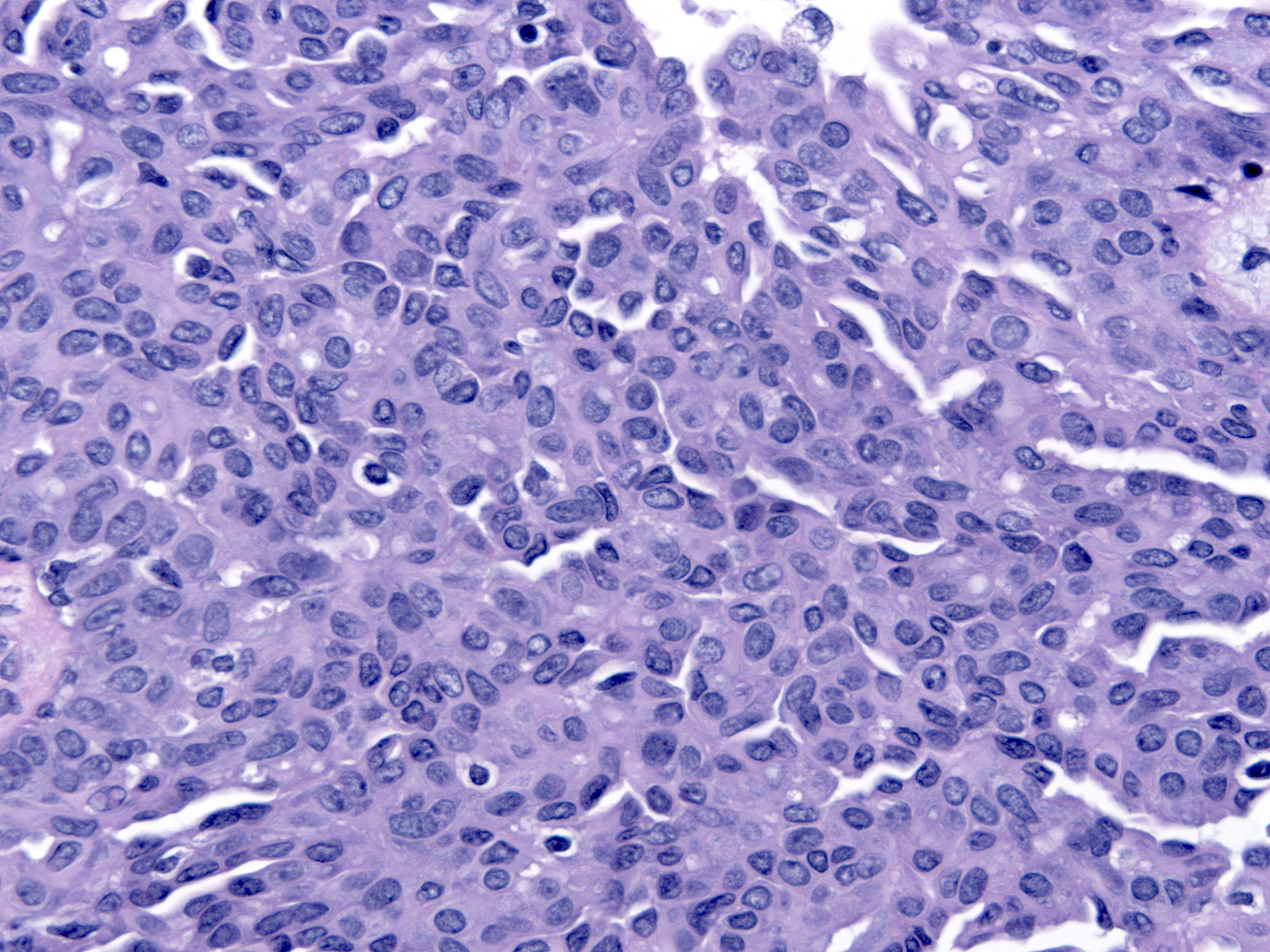 |
|
|
| The nuclei often have folds, notches, and grooves. |
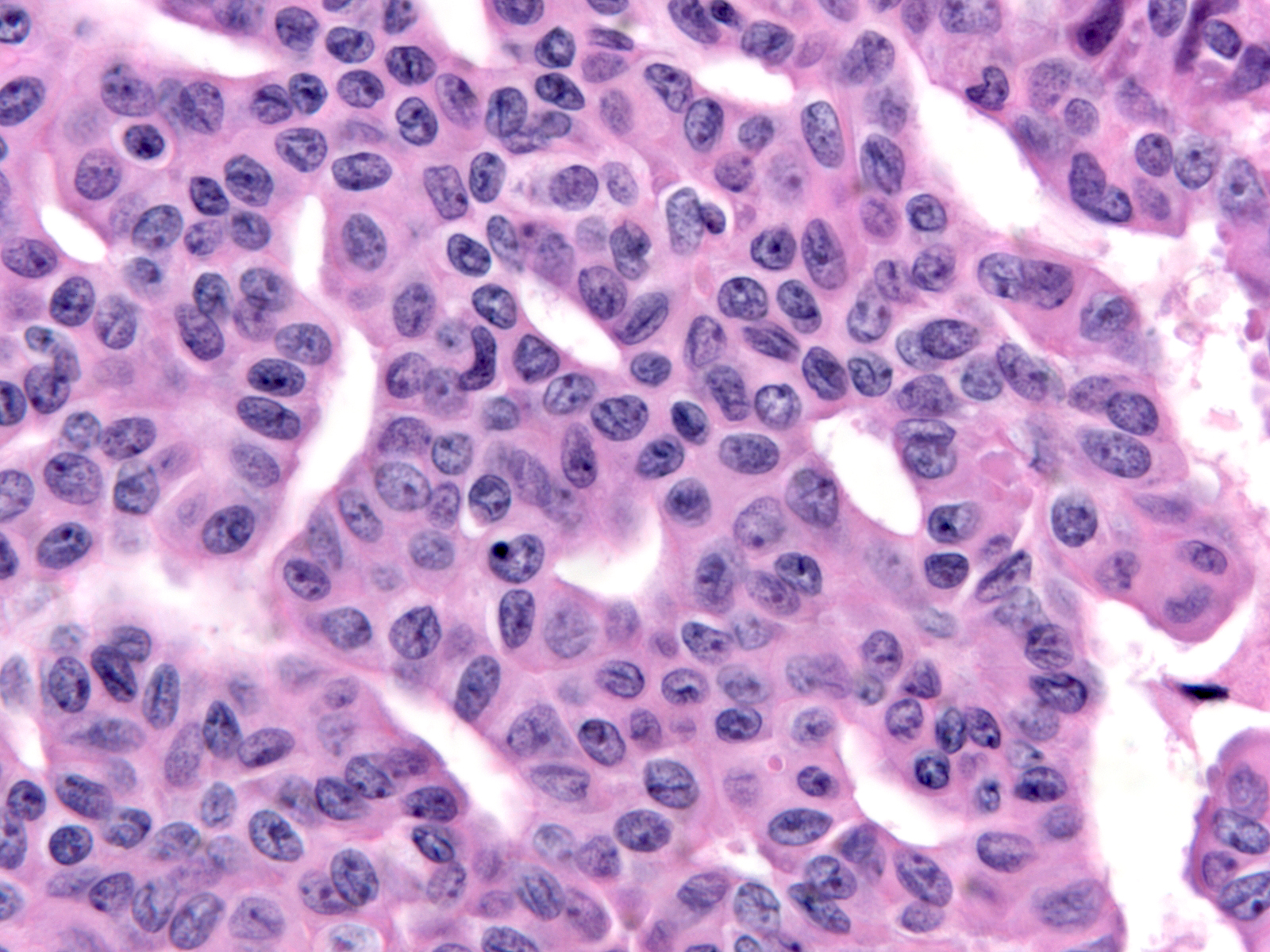 |
|
|
The chromatin pattern varies from nucleus to nucleus. Many nuclei have granular chromatin, which often shows some clearing, whereas other nuclei, usually constituting a small minority, have finely dispersed chromatin. In occasional examples, the nuclei with finely dispersed chromatin predominate.
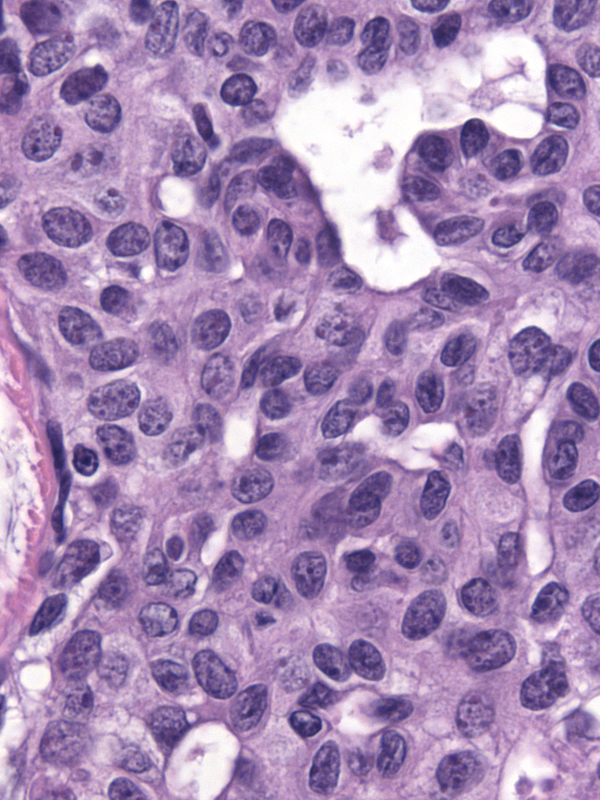 |
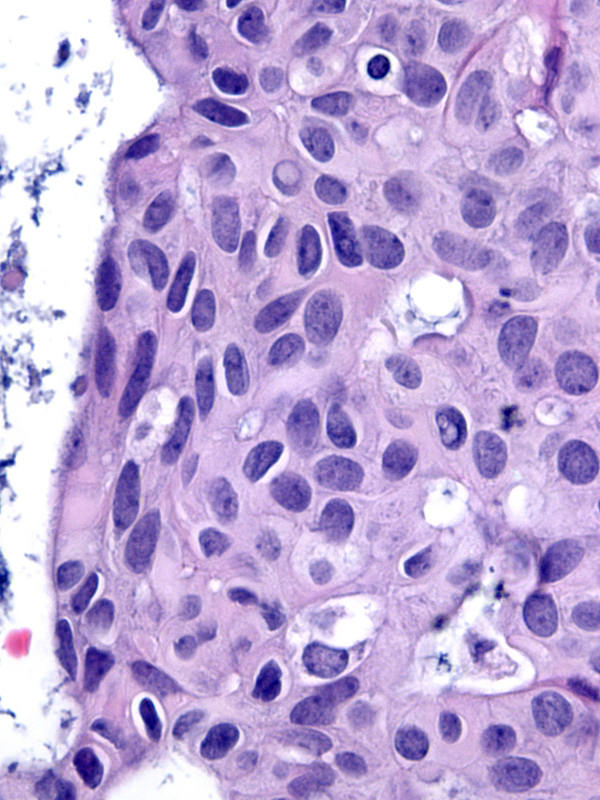 |
| Granular chromatin. |
Finely dispersed chromatin. |
| Most nuclei contain a single, easily seen but modest in size, round or oval nucleolus. |
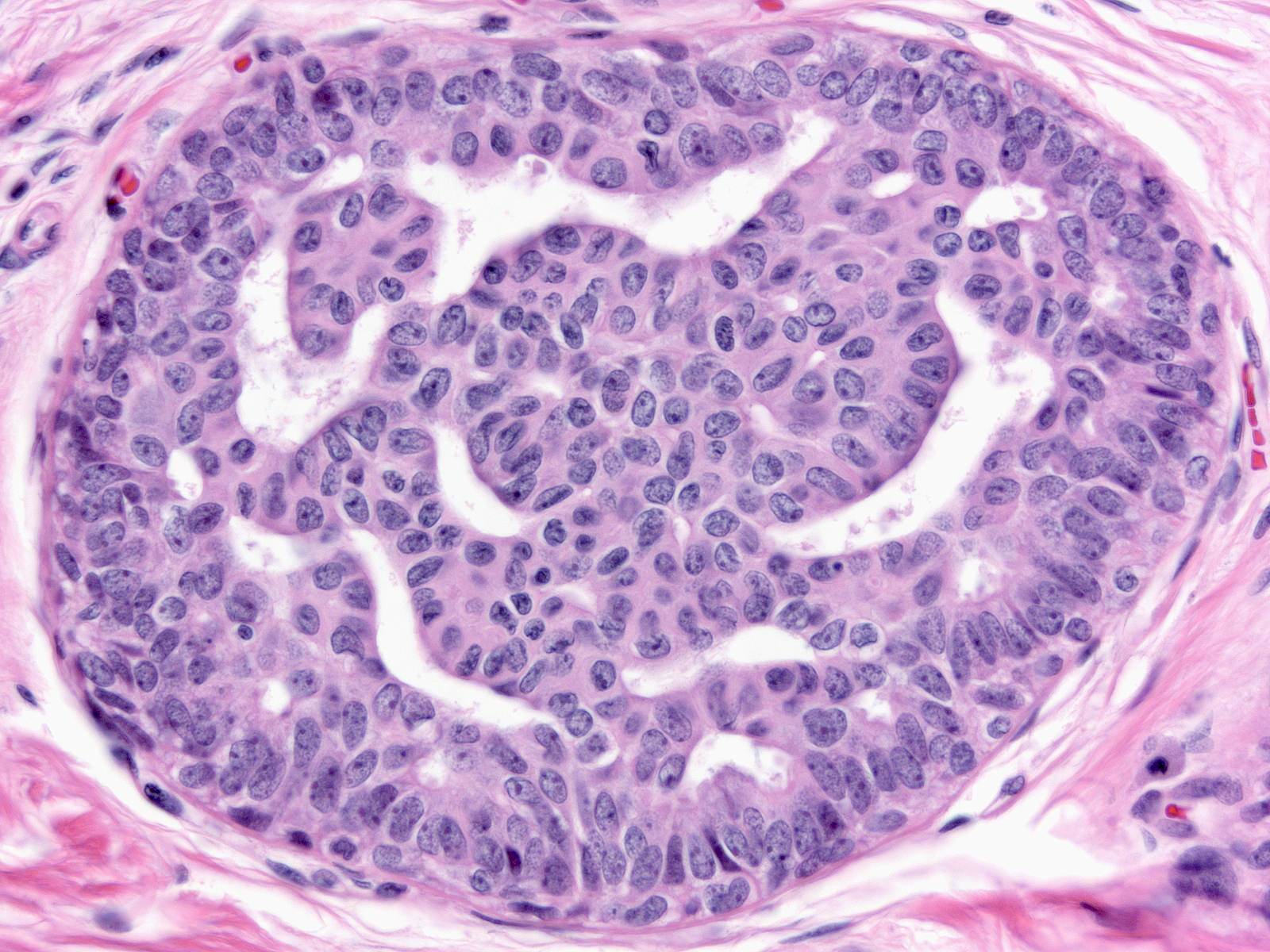 |
|
|
| The nuclei sometimes contain eosinophilic inclusion bodies of an unknown nature. |
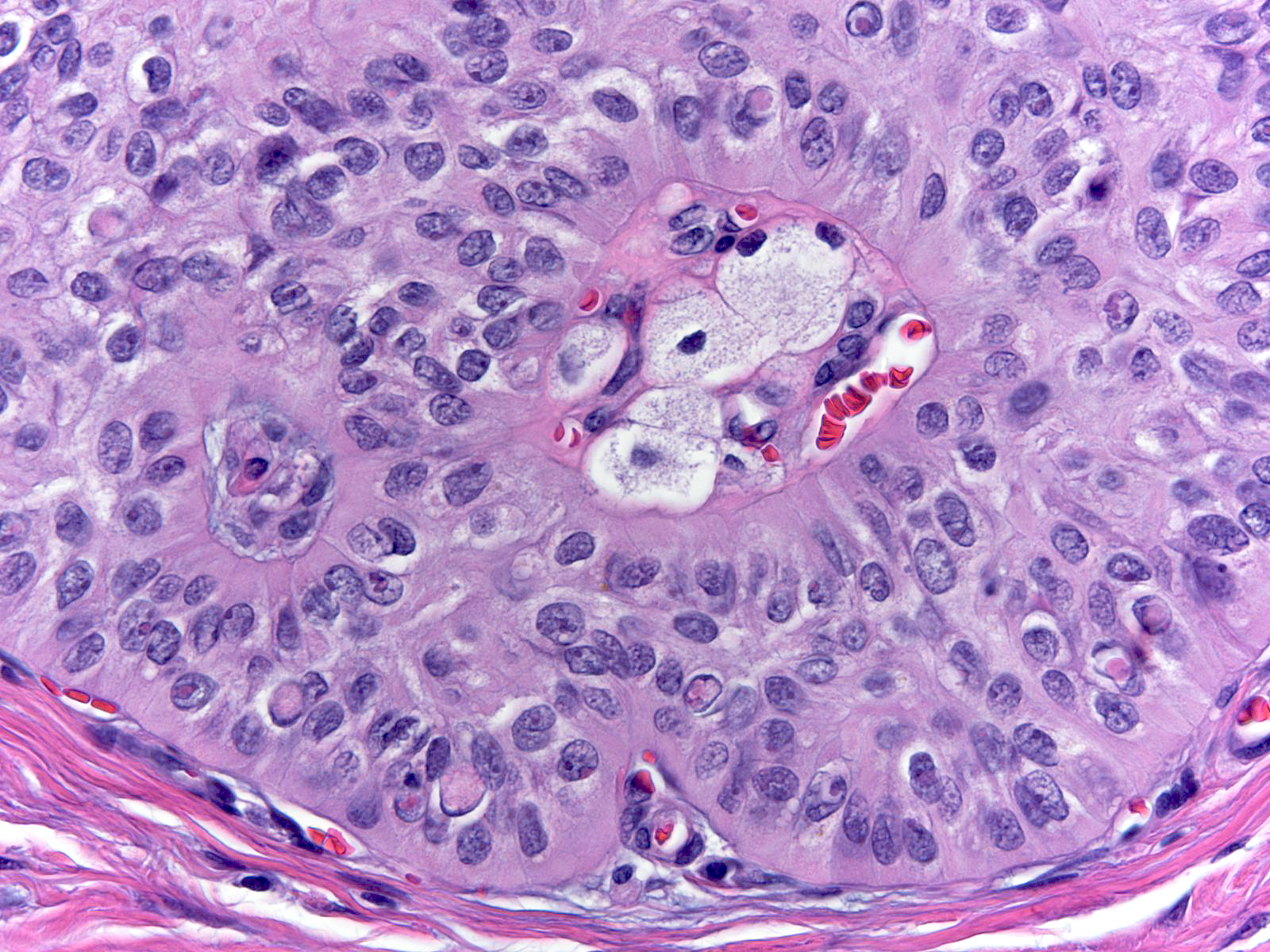 |
|
|
The presence of these inclusions offers helpful diagnostic information when considered in the proper context. Many examples of usual ductal hyperplasia lack these inclusions, so their absence does not exclude that diagnosis. On the other hand, low‑grade ductal carcinomas with the exception of the endocrine varieties do not possess this type of intranuclear inclusion; consequently, their presence essentially excludes the diagnosis of conventional low‑grade ductal carcinoma in-situ. The cells in high‑grade ductal carcinoma in‑situ and atypical apocrine proliferations can contain inclusions, but one would not consider either diagnosis for a lesion resembling usual ductal hyperplasia. Irradiated benign cells can also contain intranuclear inclusions, but other cytological features of radiation usually make that diagnosis clear.
| Detecting intranuclear inclusions proves especially useful when analyzing cauterized epithelium, for they usually persist despite the cauterization. A cauterized or traumatized epithelium in which one can identify eosinophilic intranuclear inclusion most probably represents usual ductal hyperplasia, especially if it occupies a region containing other foci of well preserved hyperplastic ductal cells. |
 |
|
|
The cytoplasm varies from eosinophilic (left) to amphophilic (right); almost never does it appear clear.
| Hyperplastic cells occasionally undergo apocrine change. |
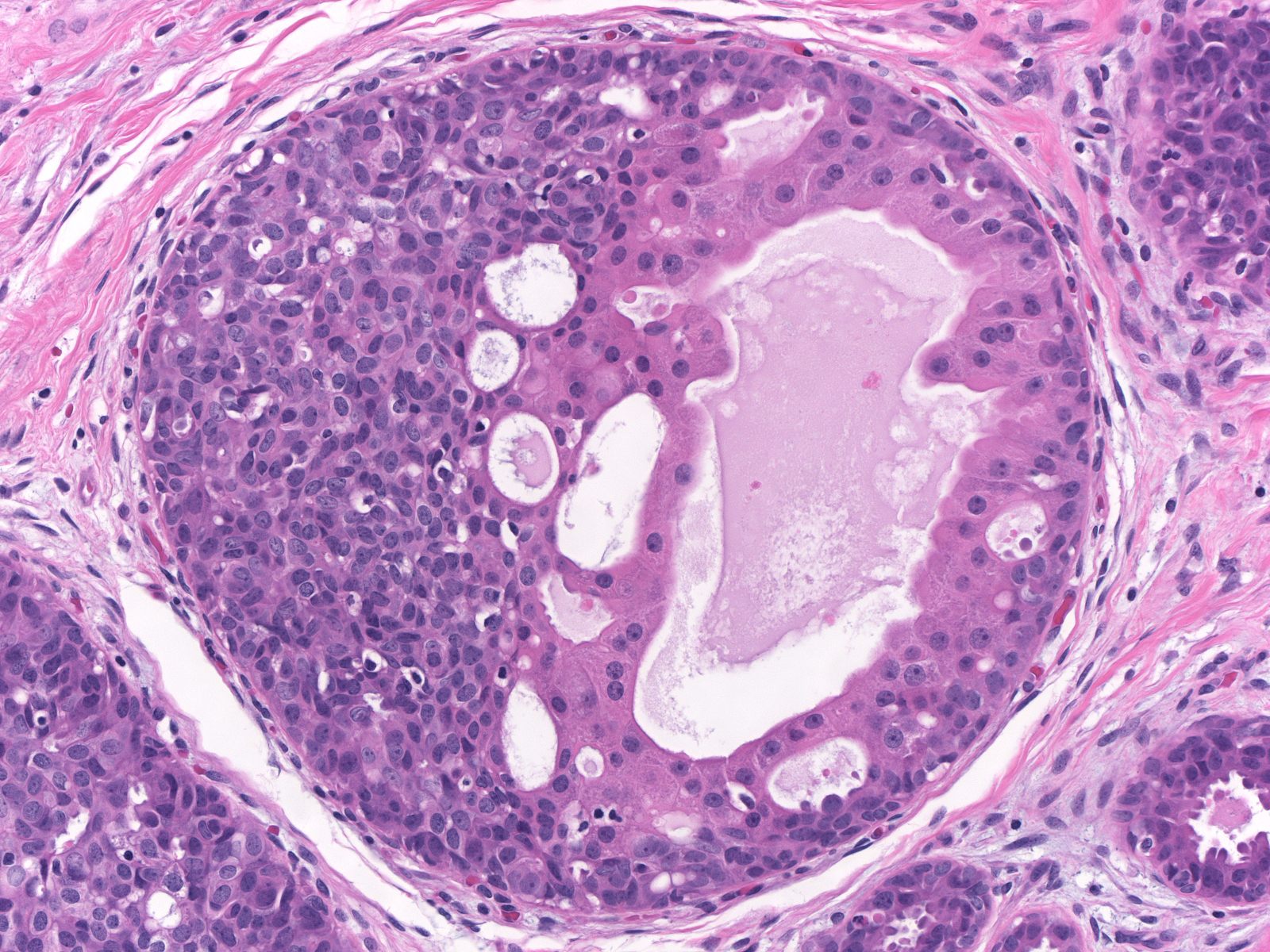 |
|
|
Hyperplastic ductal cells do not ordinarily produce calcifications or mucin, but one can observe either in rare cases. In these circumstances, the calcifications or the mucin usually arose in other lesions and became engulfed by the hyperplastic population.
| Staining of stratified forms of usual ductal hyperplasia for CK5/6 demonstrates a mixture of positive and negative cells. The majority stains strongly. Flat forms of usual ductal hyperplasia usually do not stain for the protein. |
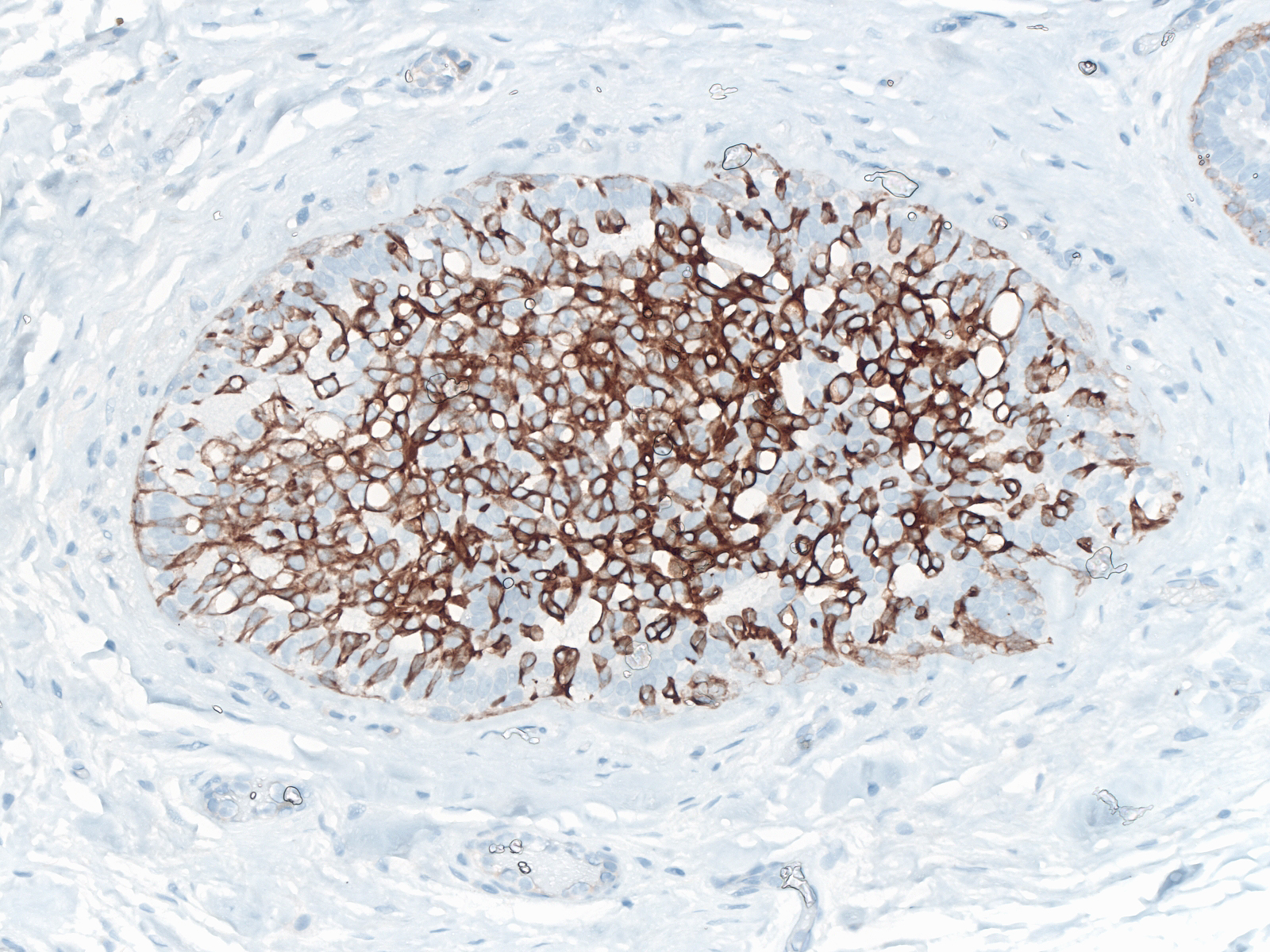 |
|
|
Florid UDH
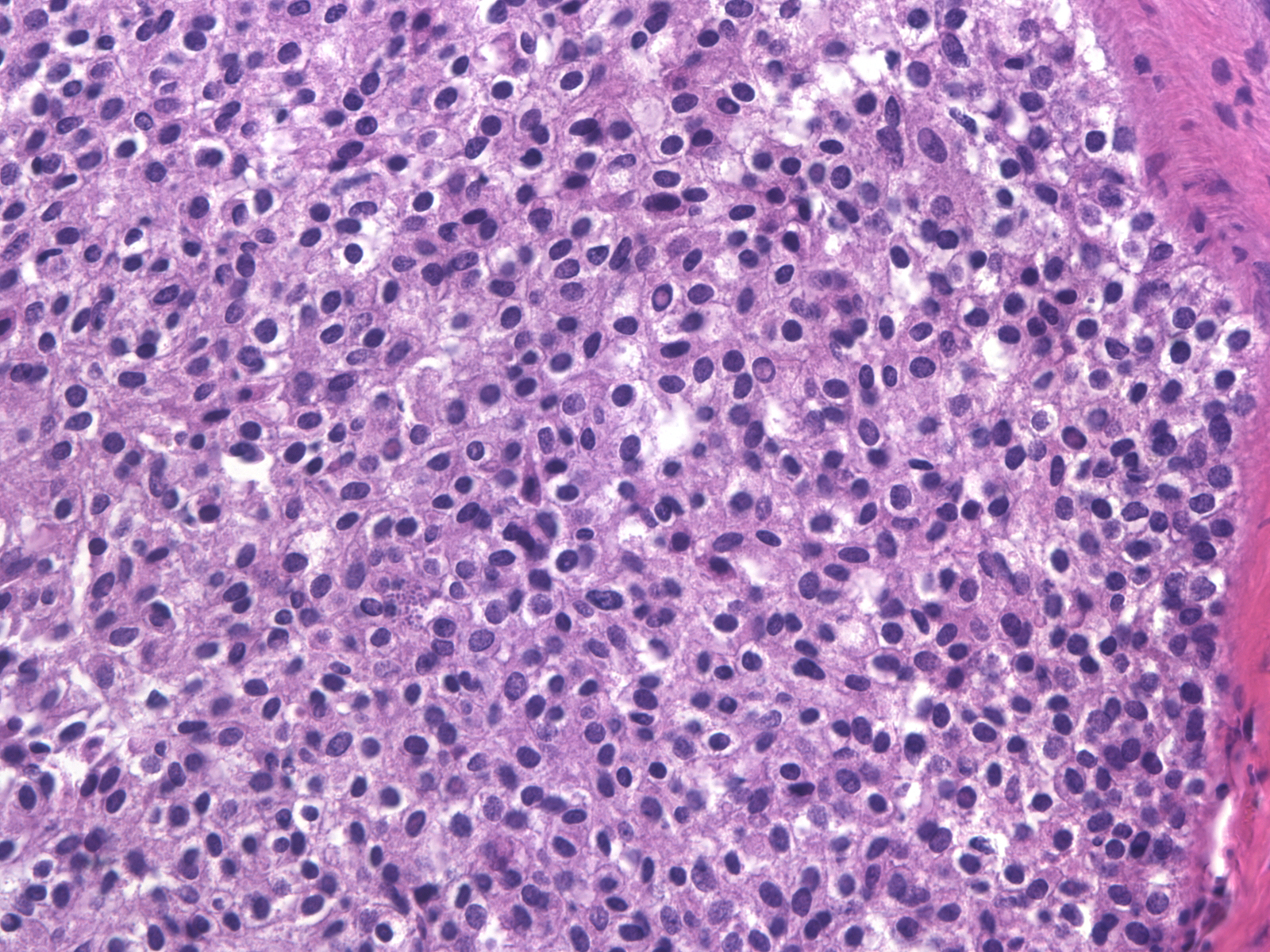
| Although the presence of necrosis most commonly reflects a malignant proliferation, the central cells in foci of florid usual ductal hyperplasia can demonstsrate this phenomenon. |
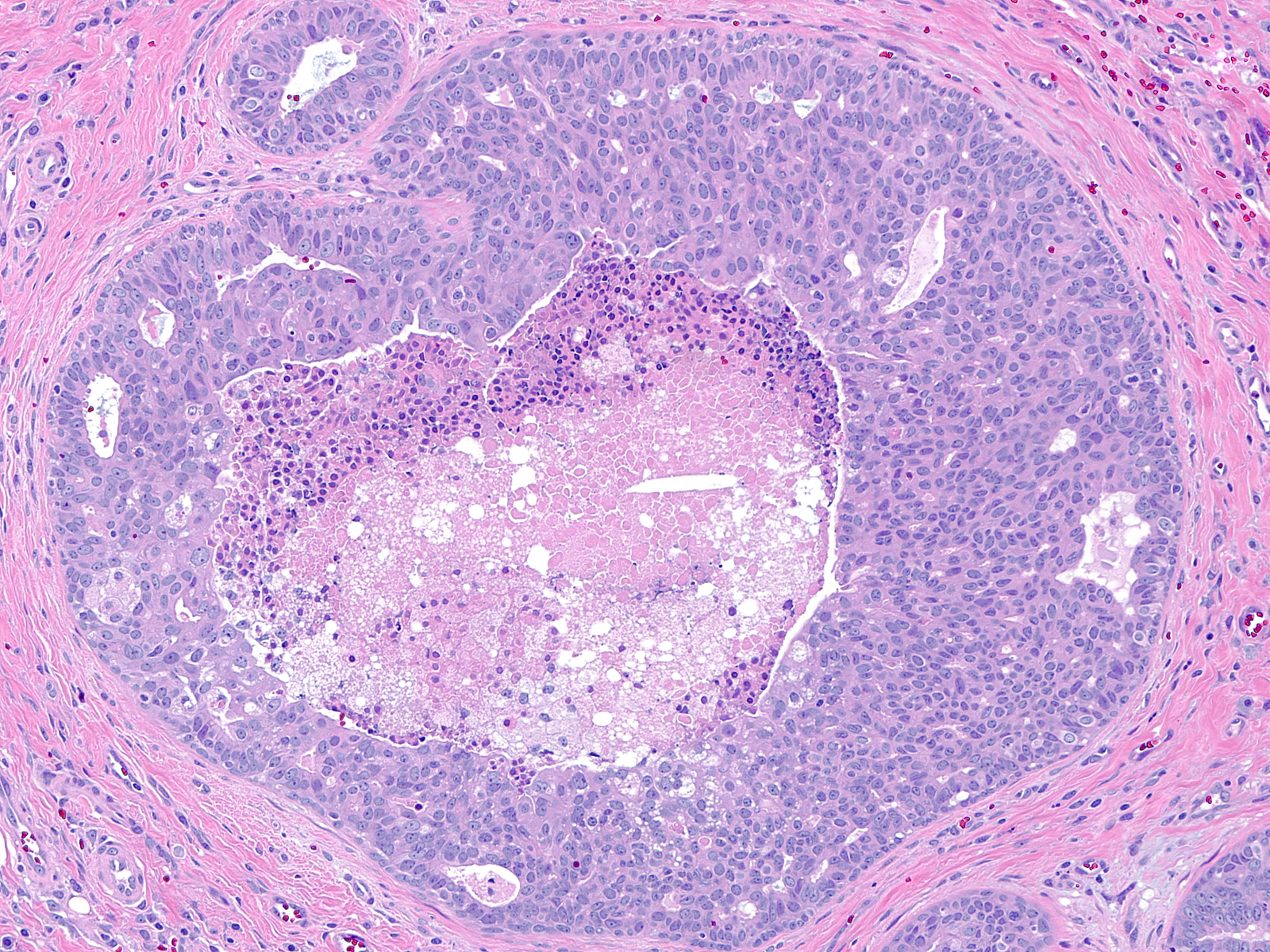 |
|
|
| Necrosis in UDH occurs most commonly in the proliferative zone of radial scars. Attention to the cytological characteristics of the proliferative cells identifies the population as benign. |
 |
|
|
Foci of florid UDH can also contain rare normal‑appearing mitotic figures in cells near the basement membrane, but one does not observe atypical mitotic figures. If all other features of UDH are identified, the presence of necrosis or mitotic figures need not detract from the diagnosis of UDH.
Breast Pathology