General
Radiation induced changes can cause alarm, but they have characteristic features.
- Definition: Radiation changes arise from therapeutic irradiation of the breast.
- Clinical Significance: Radiation changes do not usually cause clinical findings. On occasion, irradiation can lead to the formation of calcifications evident by mammography. Most often, the pathologist encounters the effects of irradiation superimposed on a clinically significant abnormality.
- Microscopic Findings: Glandular atrophy and spotty epithelial atypia constitute the characteristic changes caused by irradiation. One can also occasionally observe atypia of stromal cells, hyalinization of blood vessels, epidermal atrophy, and atypia of sweat glands.
- Differential Diagnosis: High-grade ductal carcinoma in-situ (evidence of proliferation)
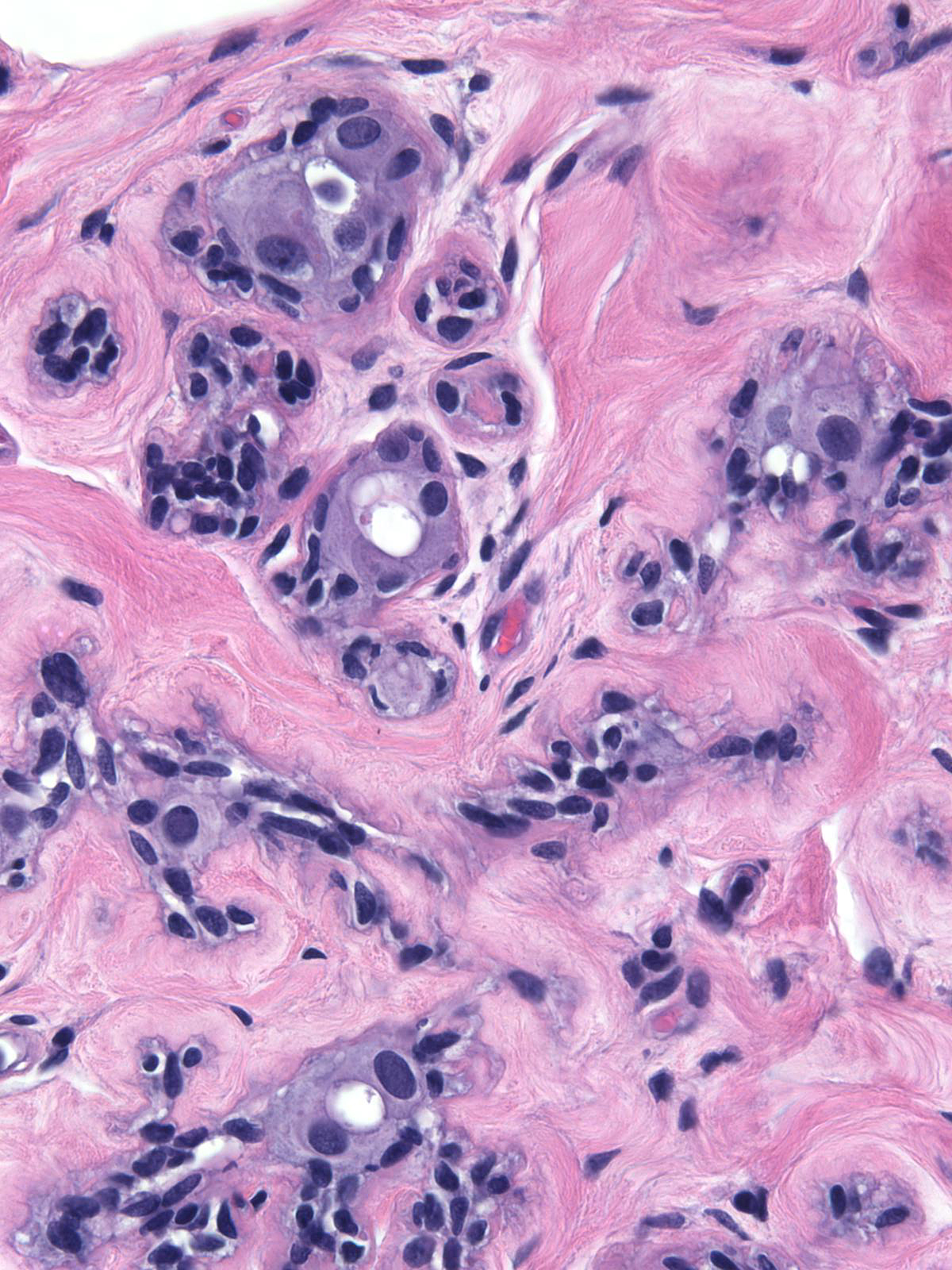
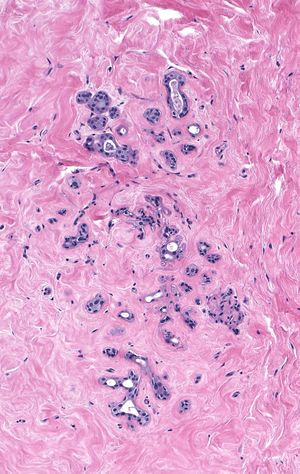
Irradiation-induced atrophy appears most obvious when it affects lobules. One observes shrinkage of the acini and sometimes their complete disappearance.
 |
 |
| Pre-irradiation |
Post-irradiation |
| Scattered epithelial cells demonstrate atypia. The luminal cells appear large and they possess large, pleomorphic nuclei. |
 |
|
|
The chromatin usually looks granular and somewhat pale, but it can also appear smudgy and dark.
| The irradiated nucleoli vary in their size, and the nuclei occasionally contain cytoplasmic inclusions. |
 |
|
|
| The cytoplasm often contains small vacuoles, which make it look foamy. The myoepithelial cells typically appear prominent, possibly because collapse of the acini packs them together closely. The myoepithelial cell nuclei stain darkly. |
 |
|
|
| The basement membranes appear thick. Small spaces that can form between the basement membrane and the myoepithelial cells give the subepithelial zone a moth-eaten appearance. |
 |
|
|
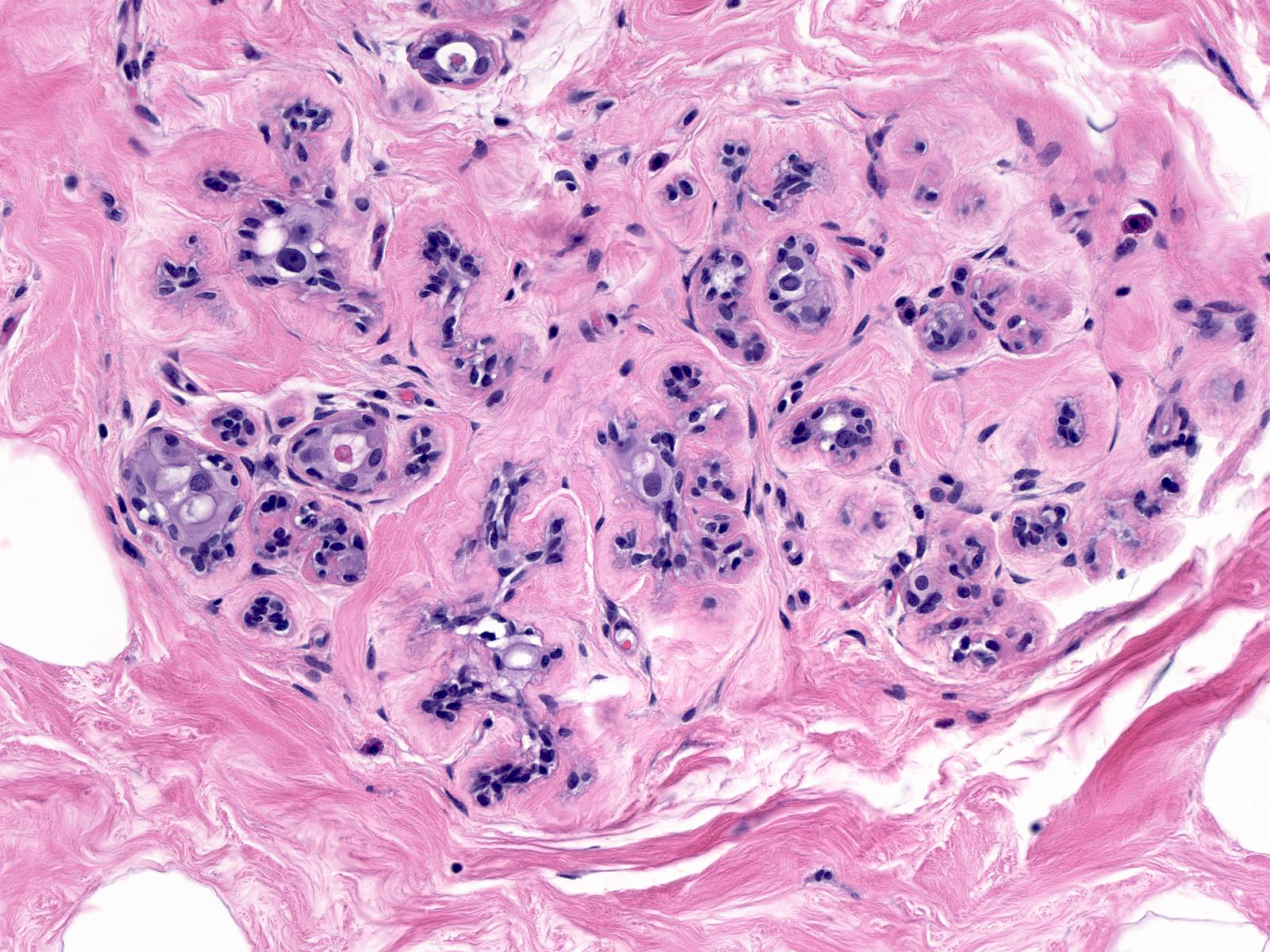
The alterations seen in irradiated small ducts duplicate those seen in lobules: shrinkage in caliber and scattered epithelial atypia.
| Irradiation does not affect the breast uniformly. Even within small regions, one sees variation in the extent of the changes. |
 |
|
|
| The degree of the changes also varies with time. One tends to see the greatest number of atypical luminal cells during the first one or two years after radiation. During this period, the cells can display striking pleomorphism. (Changes less than 2 years post-irradiation) |
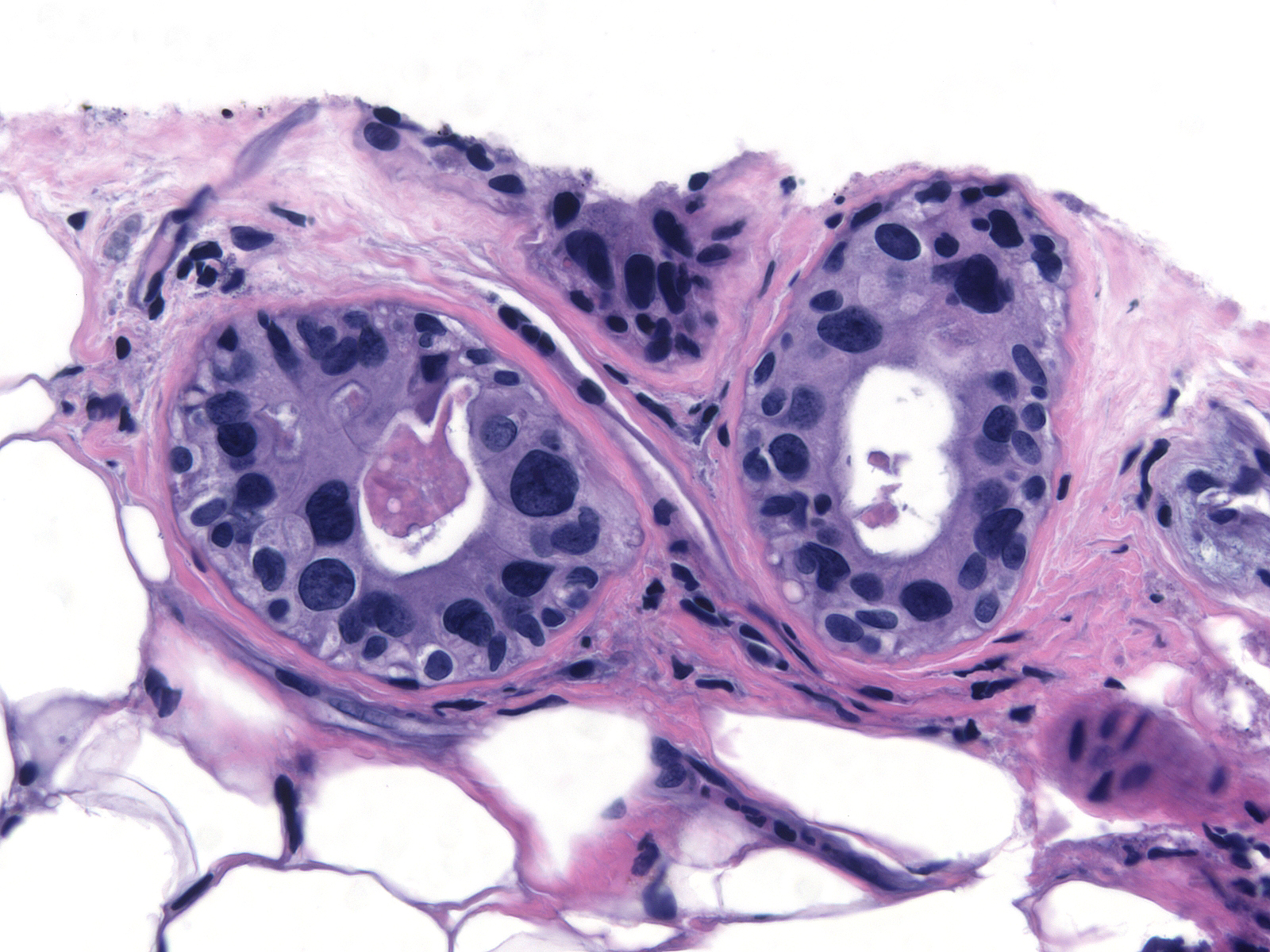 |
|
|
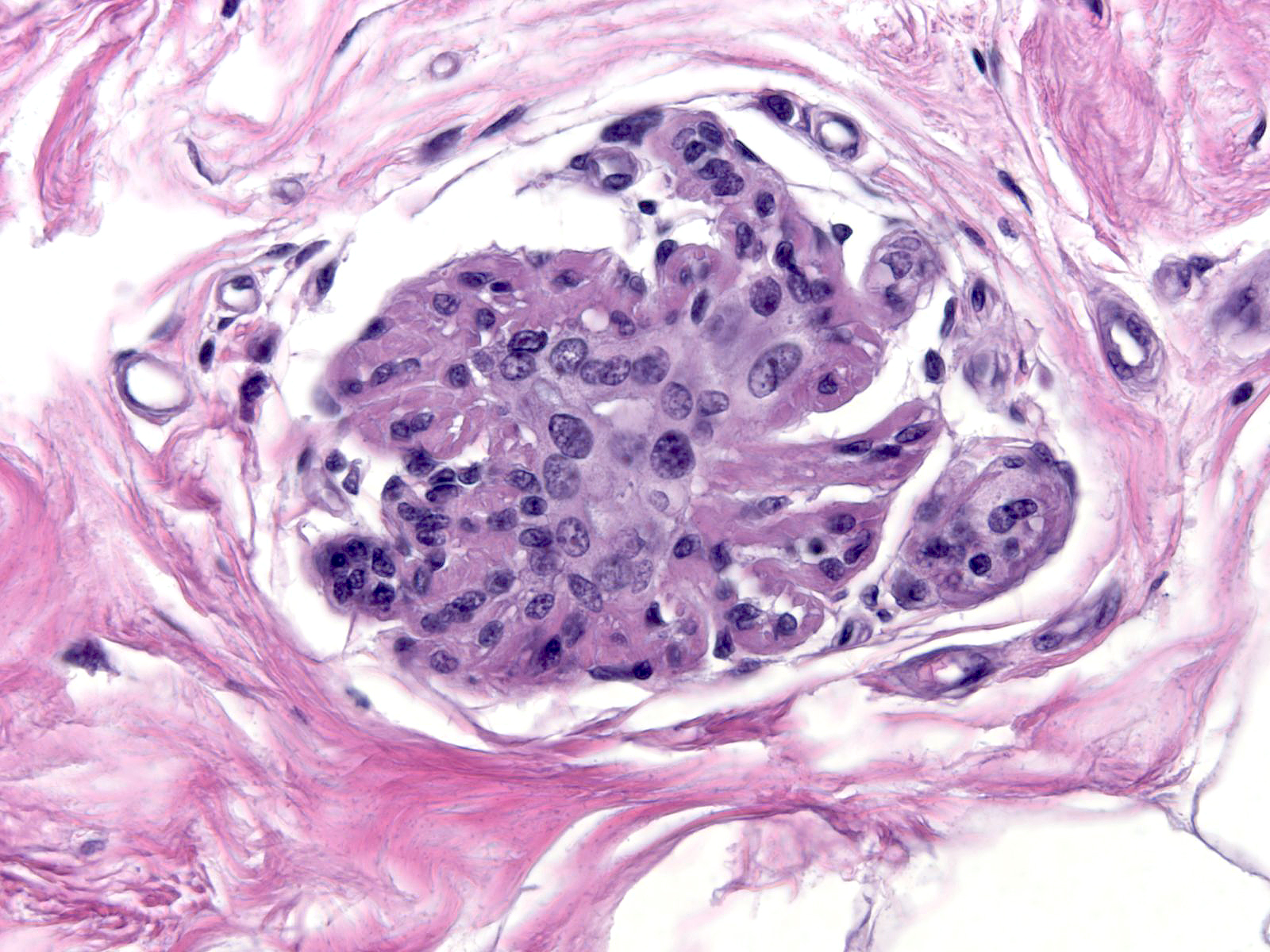
| As time passes, the number of atypical cells diminishes, but the atypical cytological features remain. One can always find at least a few cells showing radiation changes. (Changes 17 years post-irradiation) |
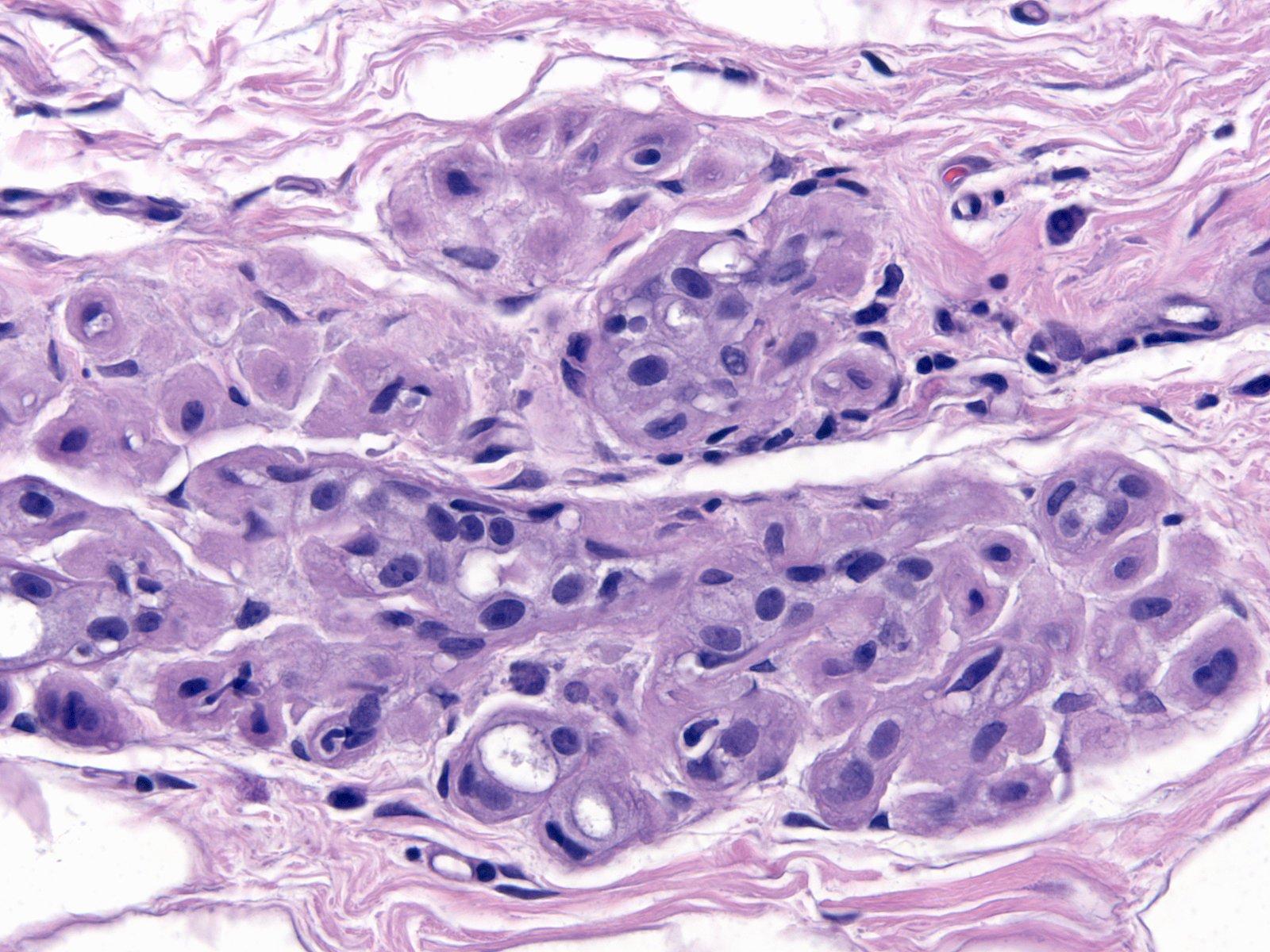 |
|
|
Distinguishing irradiated benign cells from those of low‑grade ductal carcinoma in-situ does not pose a problem. The degree of cellular and nuclear pleomorphism exhibited by irradiated cells exceeds that shown by the cells of low‑grade ductal carcinoma in-situ. To distinguish irradiated benign cells from high‑grade carcinoma cells, one must consider the distribution of the cells in question and look for evidence of proliferation and necrosis. Irradiated cells appear scattered singly or in small groups throughout the breast, whereas ductal carcinoma cells occupy a connected group of ducts and lobules in one region of the breast. Evidence of proliferation includes: stratification of cells, filling and distention of glands, and mitotic figures. Although irradiation leads to the death of cells, one does not observe necrosis in the setting of irradiation. On the other hand, necrosis occurs in many cases of high‑grade ductal carcinoma in-situ.
Radiation Changes of Carcinomas
Irradiated carcinoma cells show alterations similar to those seen in treated normal cells.
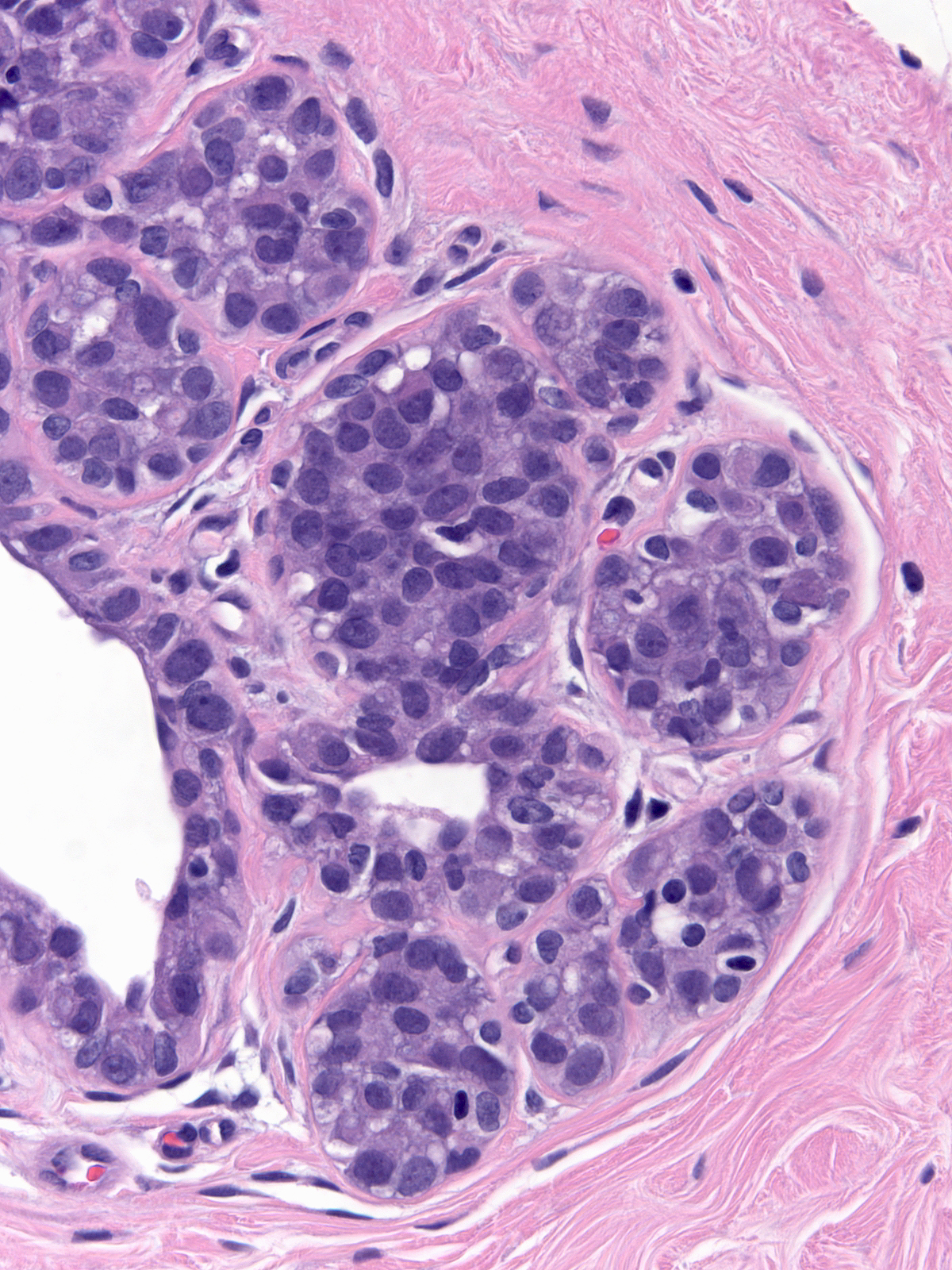 |
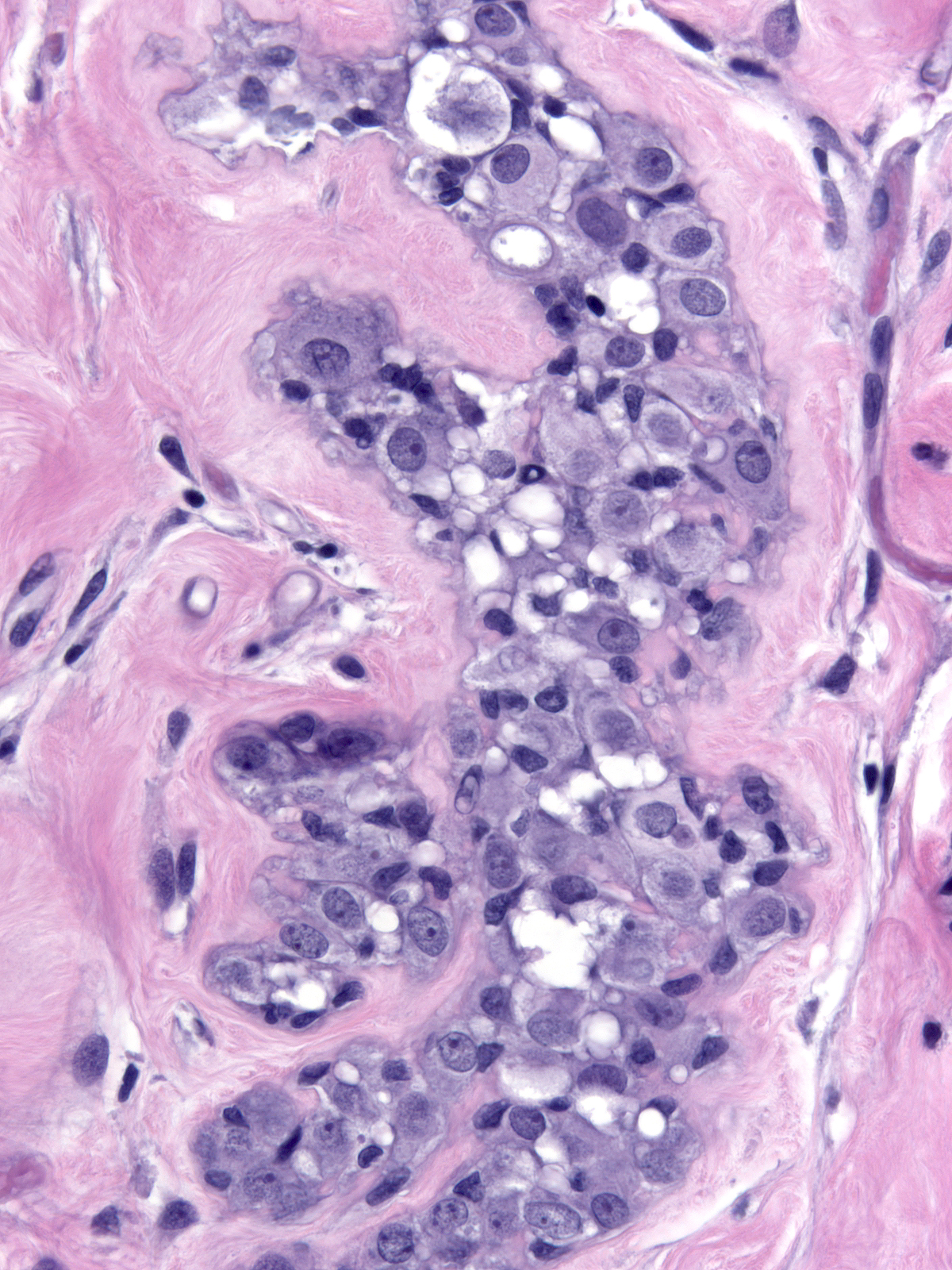 |
| Pre-irradiation LCIS |
Post-irradiation LCIS |
Epithelial Proliferations Following Irradiation
Epithelial proliferations that develop following irradiation demonstrate the same morphological features as those arising in the unirradiated breast. Recurrent carcinomas in the irradiated breast appear similar to their primary lesions. The morphological features of new carcinomas usually differ from those of the original cancers.
Breast Pathology