|
Definition: Fibrocystic changes (FCC) refers to a collection of benign changes featuring either intralobular fibrosis or cyst formation or both.
Clinical Significance: Fibrocystic changes develop in women during their reproductive years and usually regress after menopause. FCC can present on physical exam as an ill-defined mass, a region of firm tissue, or as one or more cysts. They can also present during radiographic screening as an irregular density or indeterminate calcifications.
Patients with fibrocystic changes as defined above do not experience a heightened risk for the development of breast cancer. When a core biopsy reveals fibrocystic changes, and the radiologist believes that this diagnosis explains the radiological findings, further evaluation is not undertaken.
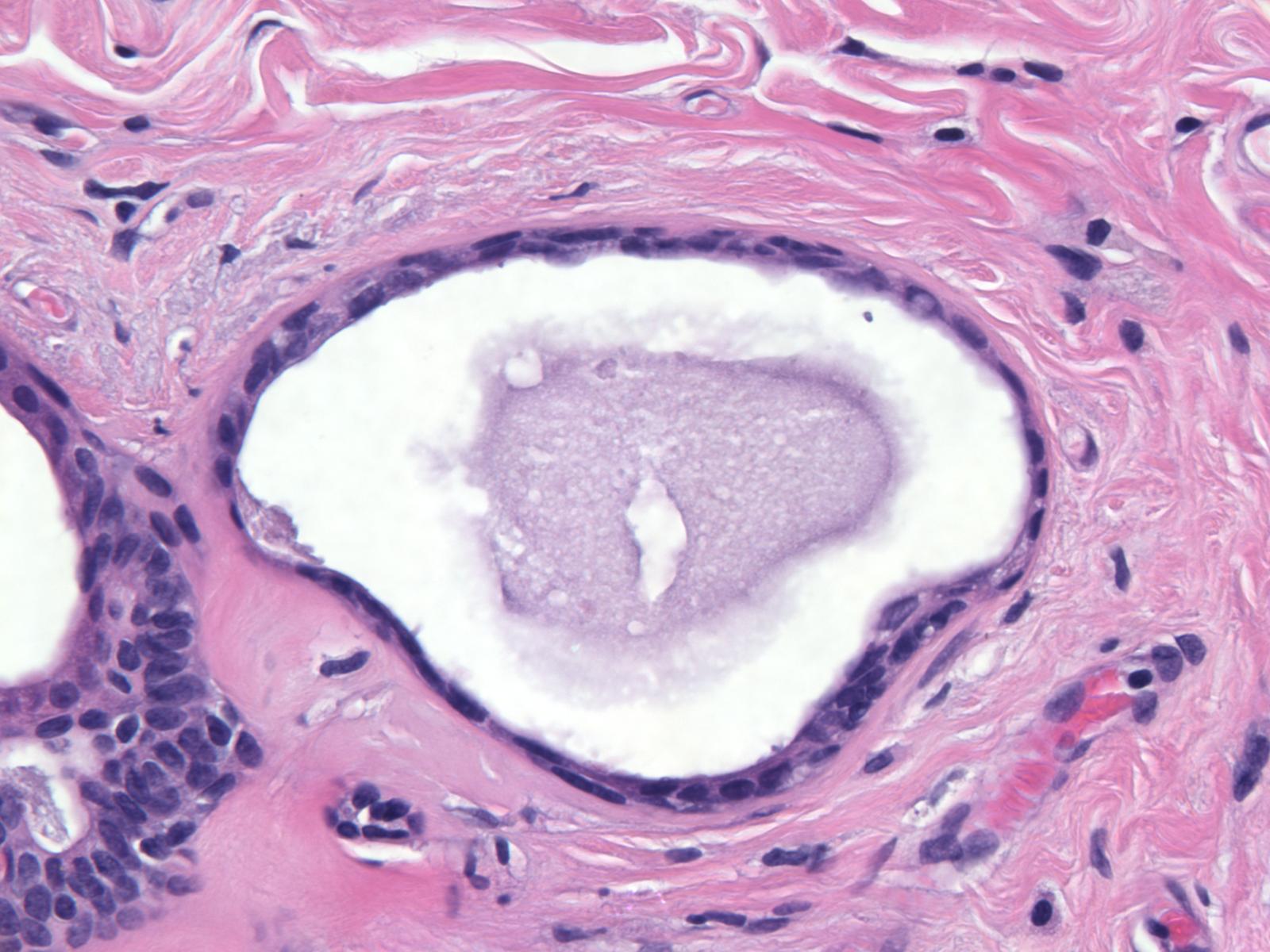
Gross Findings: Fibrocystic changes appear as a poorly defined, irregular region of firm, white tissue and/or cysts of varying size. Often the cysts and the firm white tissue mingle. The cysts contain clear, pale yellow, or red-brown fluid. Those containing red-brown fluid appear blue when they are intact and go by the name of blue dome cysts. For additional description, see the FCC portion of the basic gross pathology page.
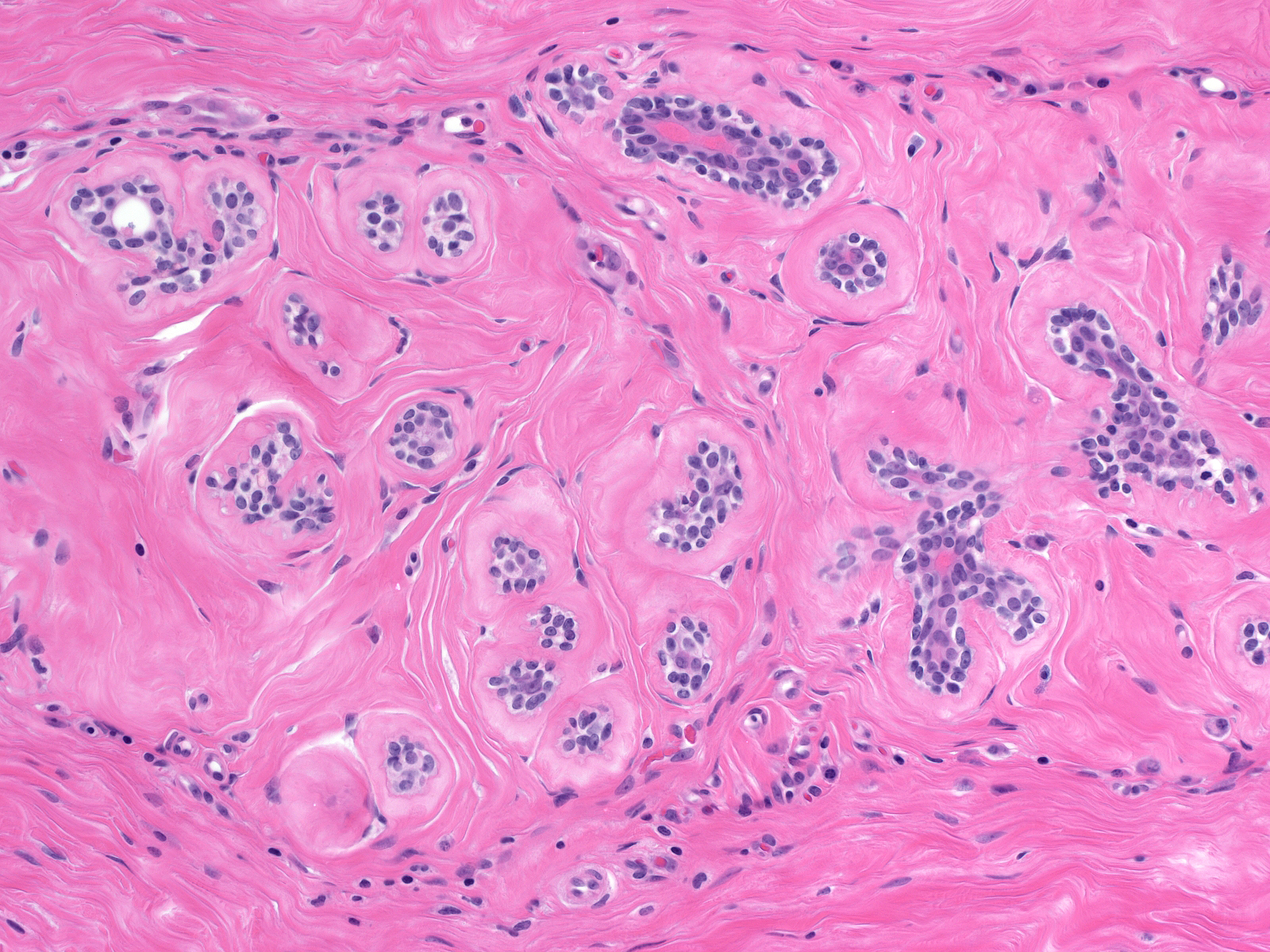
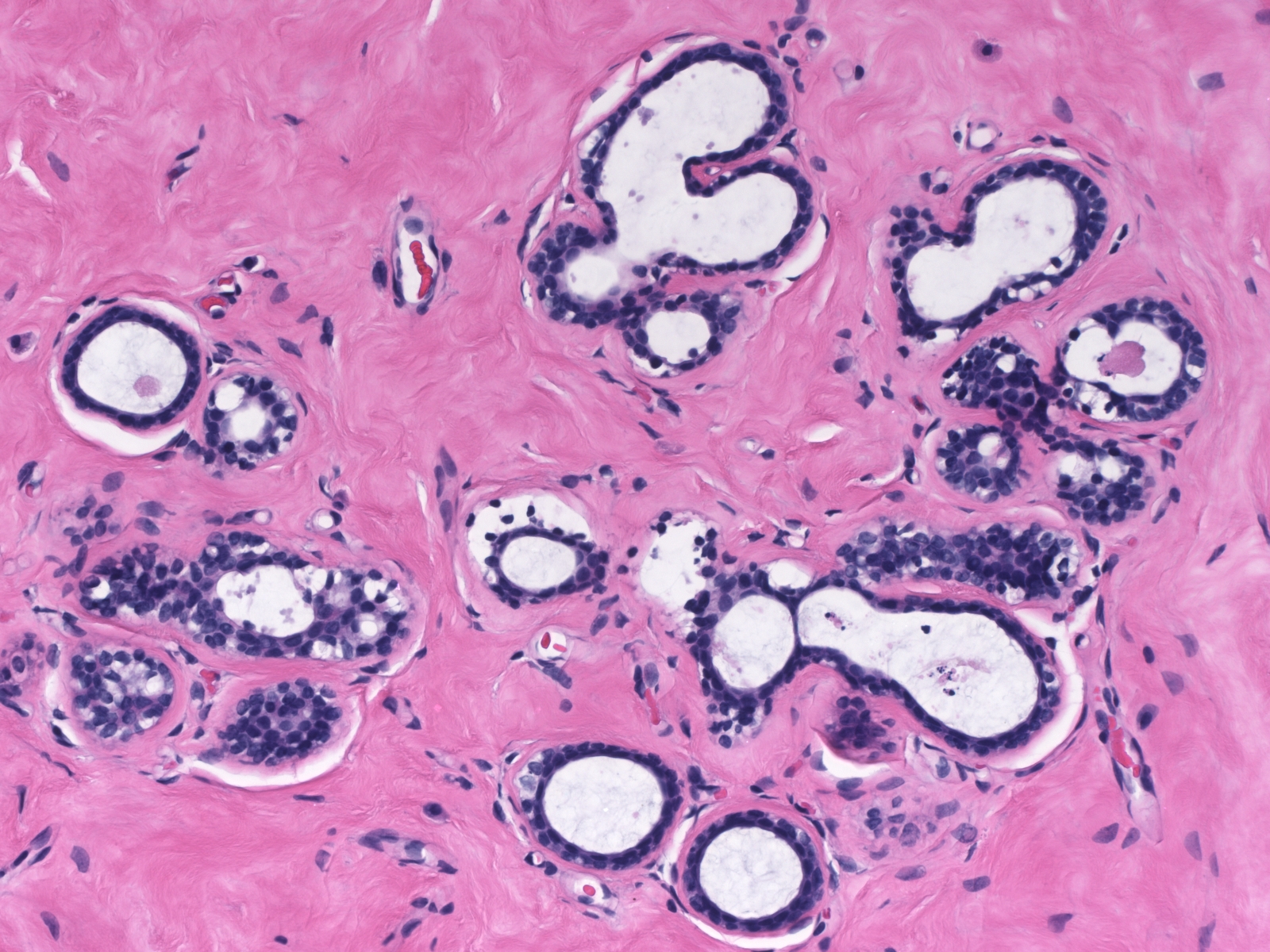
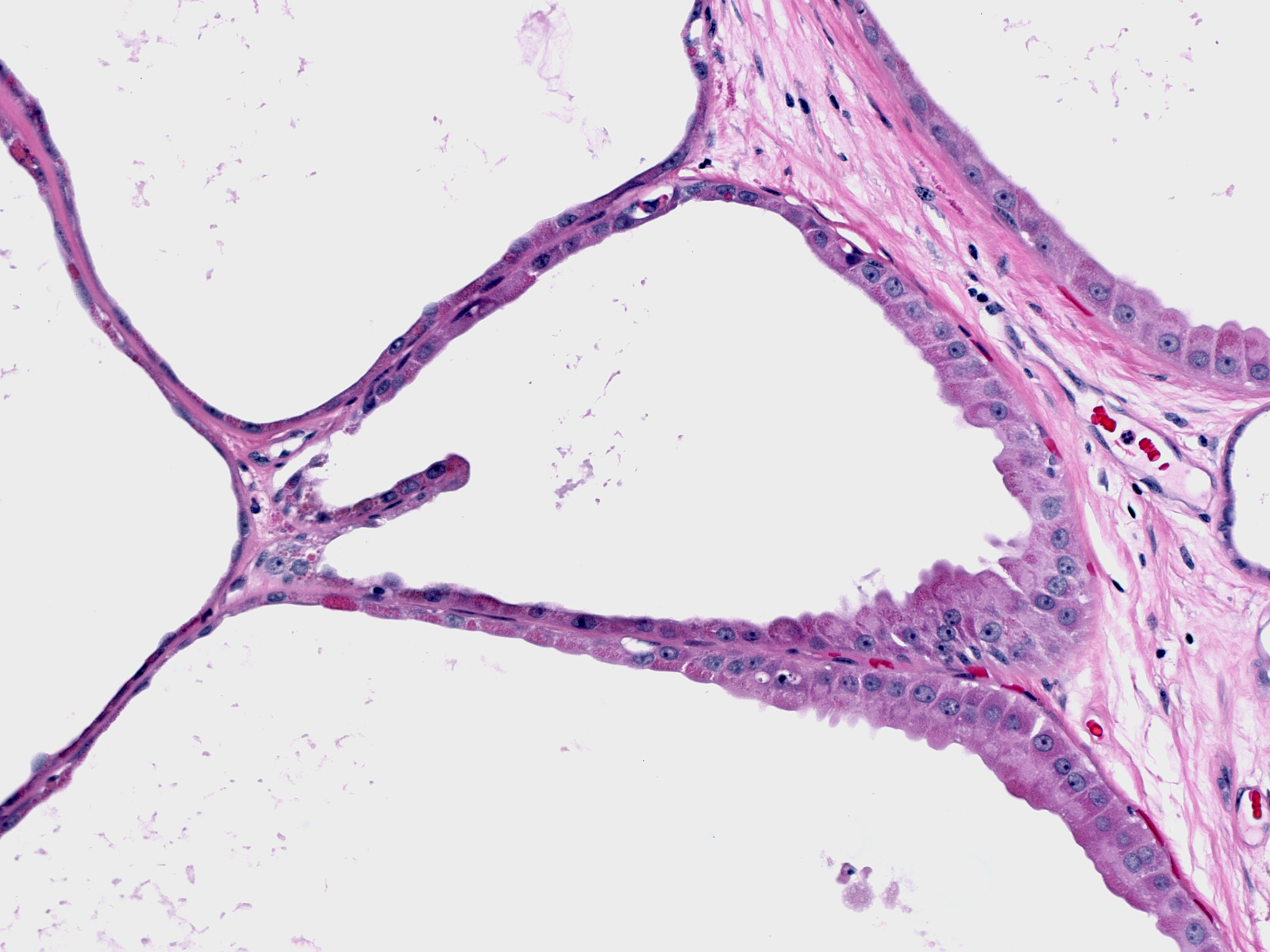
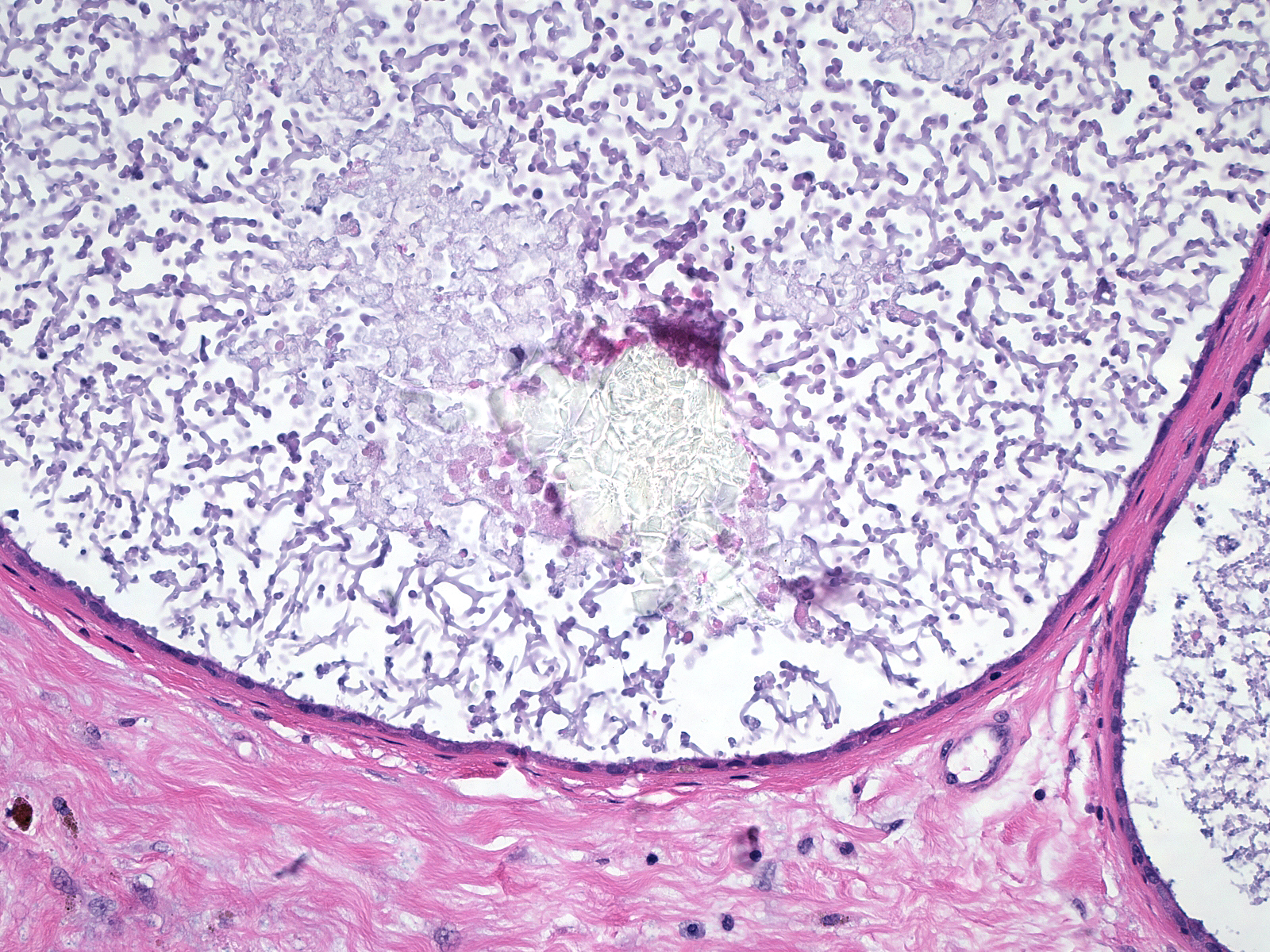
Microscopic Findings: The diagnosis of fibrocystic changes requires the presence of either intralobular fibrosis or cyst formation. Intralobular fibrosis is characterized by replacement of the specialized intralobular stroma by dense collagen. It is often accompanied by shrinkage of the associated acini and condensation of the surrounding basement membranes. The epithelial cells lining the cysts of FCC often appear flat, but they can be cuboidal or columnar. They do not appear atypical.
Differential Diagnosis: The differential diagnosis for FCC depends on the component. One could confuse cases in which fibrosis dominates with examples of pseudoangiomatous stromal hyperplasia (affects non-specialized stroma), surgical scar (usually replaces the glandular element), radiation changes (look for other radiation changes), and diabetic mastopathy. (Diabetic mastopathy is a rare lesion featuring glandular atrophy, stromal fibrosis, and dense lymphocytic infiltration.)
When the cystic component dominates one could consider such diagnoses as flat epithelial atypia (low-grade atypia), blunt duct adenosis (lush epithelial and myoepithelial cells), "clinging" carcinoma (flat, high-grade DCIS), juvenile papillomatosis (well-defined mass), and cystic hypersecretory hyperplasia (cysts containing dense, eosinophilic material). (Juvenile papillomatosis is a rare lesion in which ductal hyperplasia, apocrine metaplasia, cyst formation, and adenosis give rise to a mass. Cystic hypersecretory lesions display filling and distention of ducts and acini by colloid-like, eosinophilic secretions.)
Discussion: Pathologists differ in the histological alterations categorized as fibrocystic changes. Common teaching divides fibrocystic changes into two categories: nonproliferative (fibrosis, cysts, apocrine change, adenosis, and mild usual ductal hyperplasia) and proliferative (moderate or marked usual ductal hyperplasia, sclerosing adenosis, radial scar, and papilloma). Pathologists at MGH do not distinguish the two classes of fibrocystic changes when reporting the diagnosis, nor do they include sclerosing adenosis, radial scars, and papillomas under the rubric of fibrocystic changes. As used at MGH, the diagnosis of fibrocystic changes refers to the presence of either intralobular fibrosis or cyst formation and may also include apocrine change and usual ductal hyperplasia
|
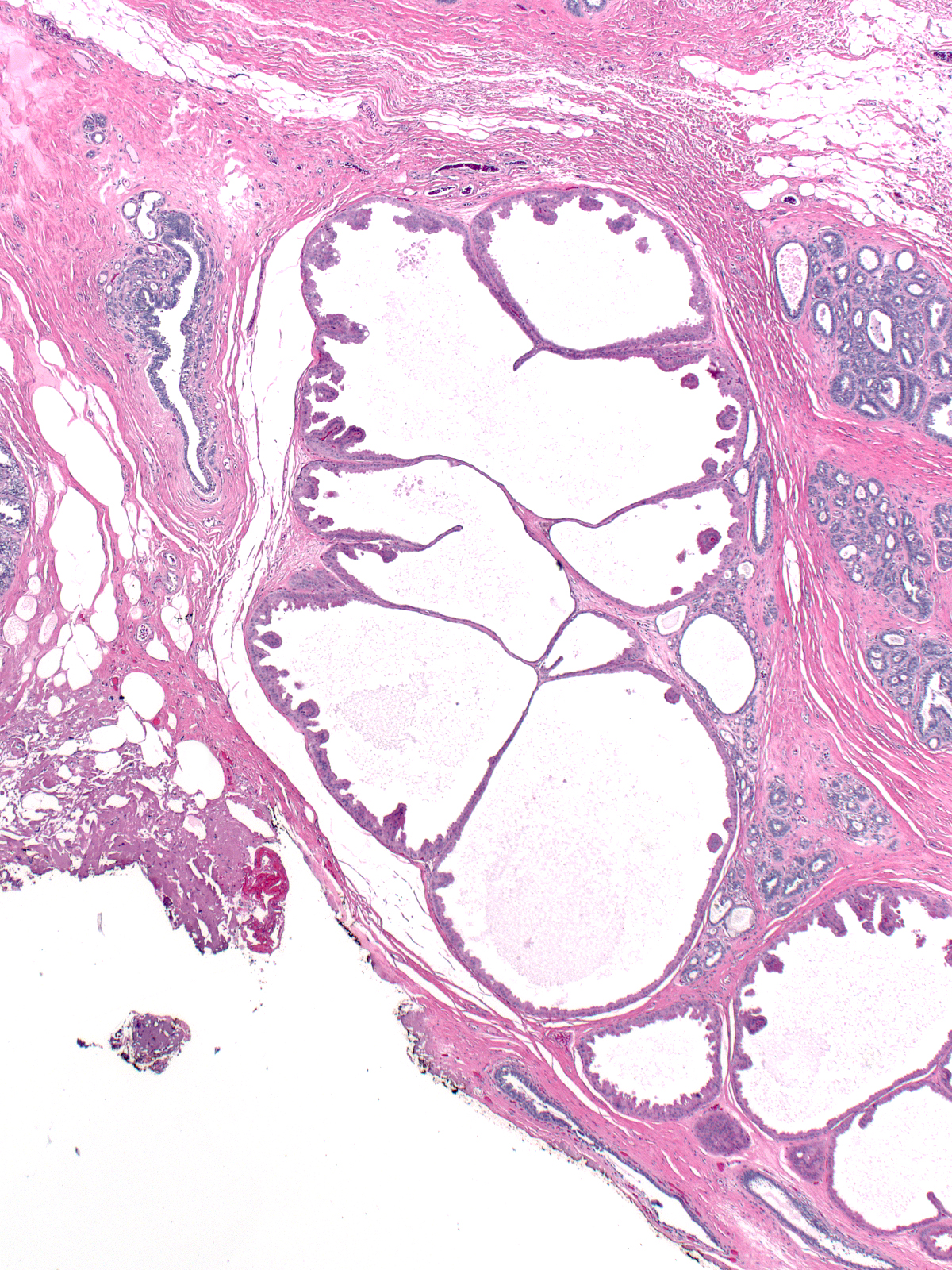 Dilatation accompanied by apocrine metaplasia transformed the acini of this lobule into a collection of microcysts. Dilatation accompanied by apocrine metaplasia transformed the acini of this lobule into a collection of microcysts. |