Introduction
|
Definition: Ductal carcinoma in-situ (DCIS) is a neoplastic proliferation of luminal type ductal epithelial cells.
Clinical Significance: Ductal carcinoma in-situ comes to clinical attention because of the presence of calcifications on a mammogram or linear enhancement on MRI. In the uncommon situation in which it produces a pronounced stromal reaction, it can present as a mass. DCIS represents the precursor to invasive ductal carcinoma and usually leads to treatment of the entire involved breast (mastectomy or excision and irradiation). Oncologists usually also recommend hormonal therapy to reduce the risk of developing carcinoma in the contralateral breast.
When a core biopsy reveals ductal carcinoma in-situ, a surgeon will typically carry out an excision of the targeted area and marginal tissue. If the radiological or the pathological findings indicate extensive involvement of the breast and in certain other circumstances, the surgeon will recommend a mastectomy and sentinel node excision rather than an excision.
Current thinking based on morphological, clinical, and genetic studies asserts that the low-grade and high-grade forms of ductal carcinoma in-situ represent distinct biological entities whose morphological precursors and genetic aberrations do not overlap.
Gross Findings: Low-grade DCIS does not usually exhibit macroscopic findings. Transected ducts of high-grade DCIS may exude yellow-white, granular or pasty material (comedonecrosis), corresponding to necrotic carcinoma cells in the duct lumen. Calcifications associated with DCIS may impart a granular texture.
Microscopic Findings: Expansive proliferation of polarized, atypical, slightly dishesive epithelial cells characterizes DCIS.
Differential Diagnosis: Usual ductal hyperplasia (highly cohesive, nonpolarized cells), atypical ductal hyperplasia (typically low-grade, less expansive), lobular carcinoma in-situ (dishesive, nonpolarized cells)
Discussion: Although the cellular features of ductal carcinoma in-situ vary from case to case and can differ somewhat within cases, four alterations represent consistent findings: cellular polarization, cellular enlargement, cytological atypia, and architectural atypia.
|
 Ductal carcinoma in-situ (top) gives rise
to invasive ductal carcinoma (bottom). Ductal carcinoma in-situ (top) gives rise
to invasive ductal carcinoma (bottom). |
Cellular Polarization
The cells of DCIS display the phenomenon of cellular polarization, a fundamental characteristic of normal epithelial cells. We recognize polarization by the location of the nucleus at one end of the cell (referred to as the basal pole) and the accumulation of cytoplasm at the opposite end (referred to as the apical cytoplasmic compartment). Normal ductal epithelial cells rely on signals associated with the basement membrane to establish the orientation needed to achieve polarity. The ability of ductal carcinoma cells to establish polarity far from the basement membrane differentiates these malignant cells from normal mammary cells and from neoplastic lobular cells.
 |
 |
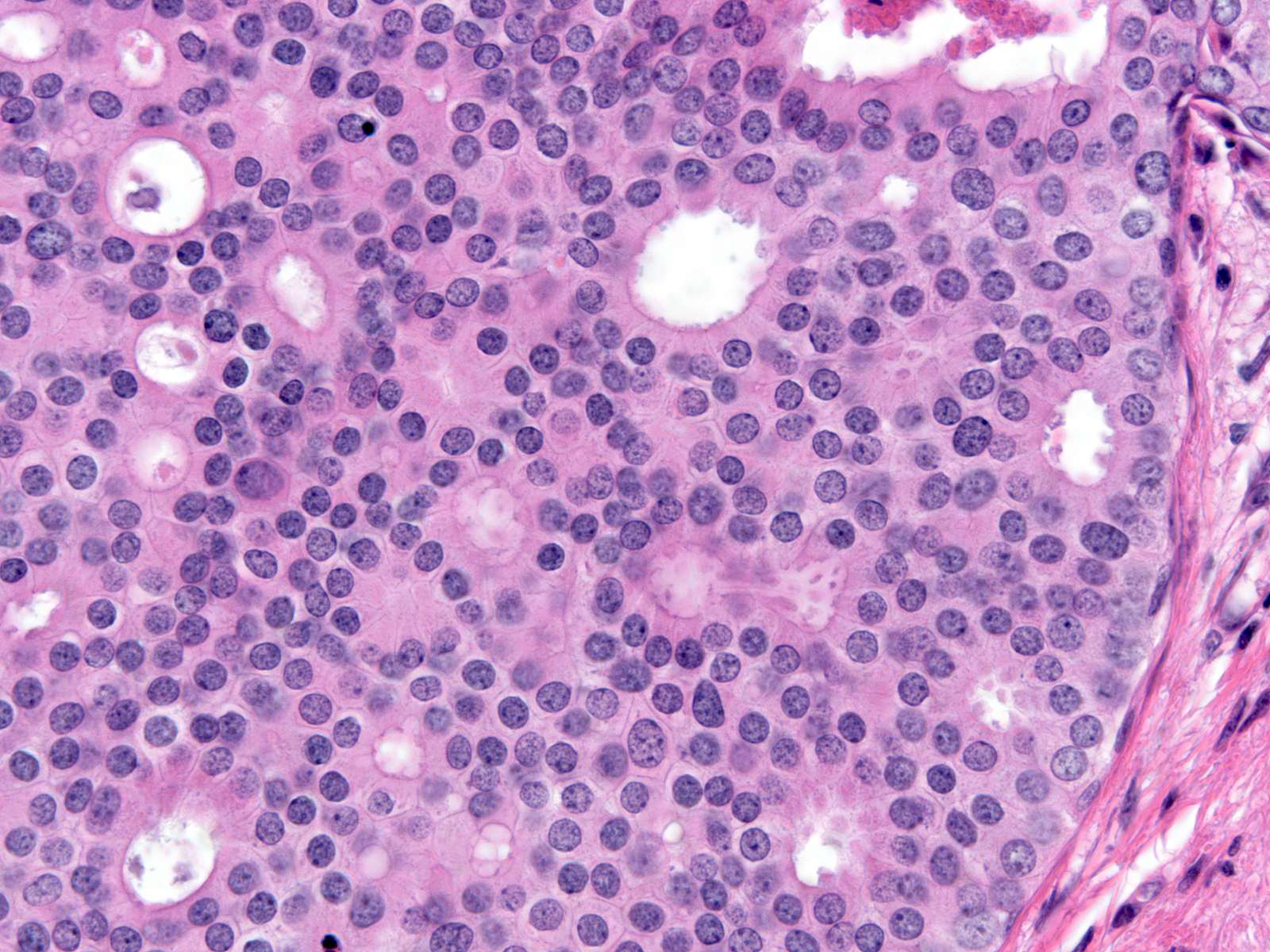
| Low-grade DCIS |
Intermediate-grade DCIS |
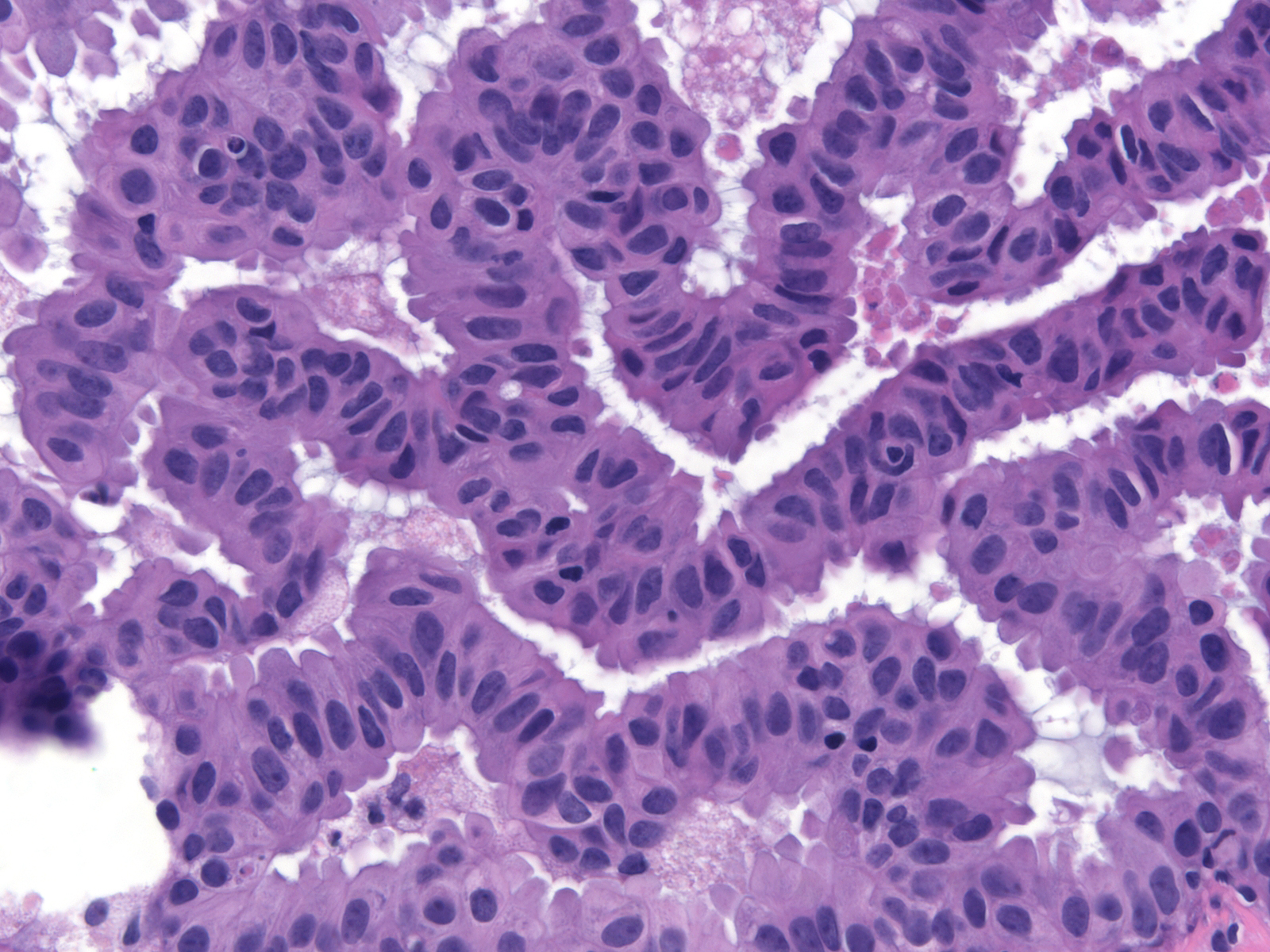
Polarized ductal cells typically display a columnar shape, an eccentric nucleus, and an apical cytoplasmic compartment. Polarization is usually obvious in the cells of low-grade DCIS (above left) and in some cases of intermediate-grade DCIS (above right); however, it may be difficult to appreciate the polarized state in other cases of intermediate-grade DCIS (below left) or in high-grade DCIS (below right). The diagnosis of these later examples of DCIS hinges on recognizing the cells' large size and pronounced cytologic atypia.
 |
 |
| Intermediate-grade DCIS |
High-grade DCIS |
Cellular Enlargement
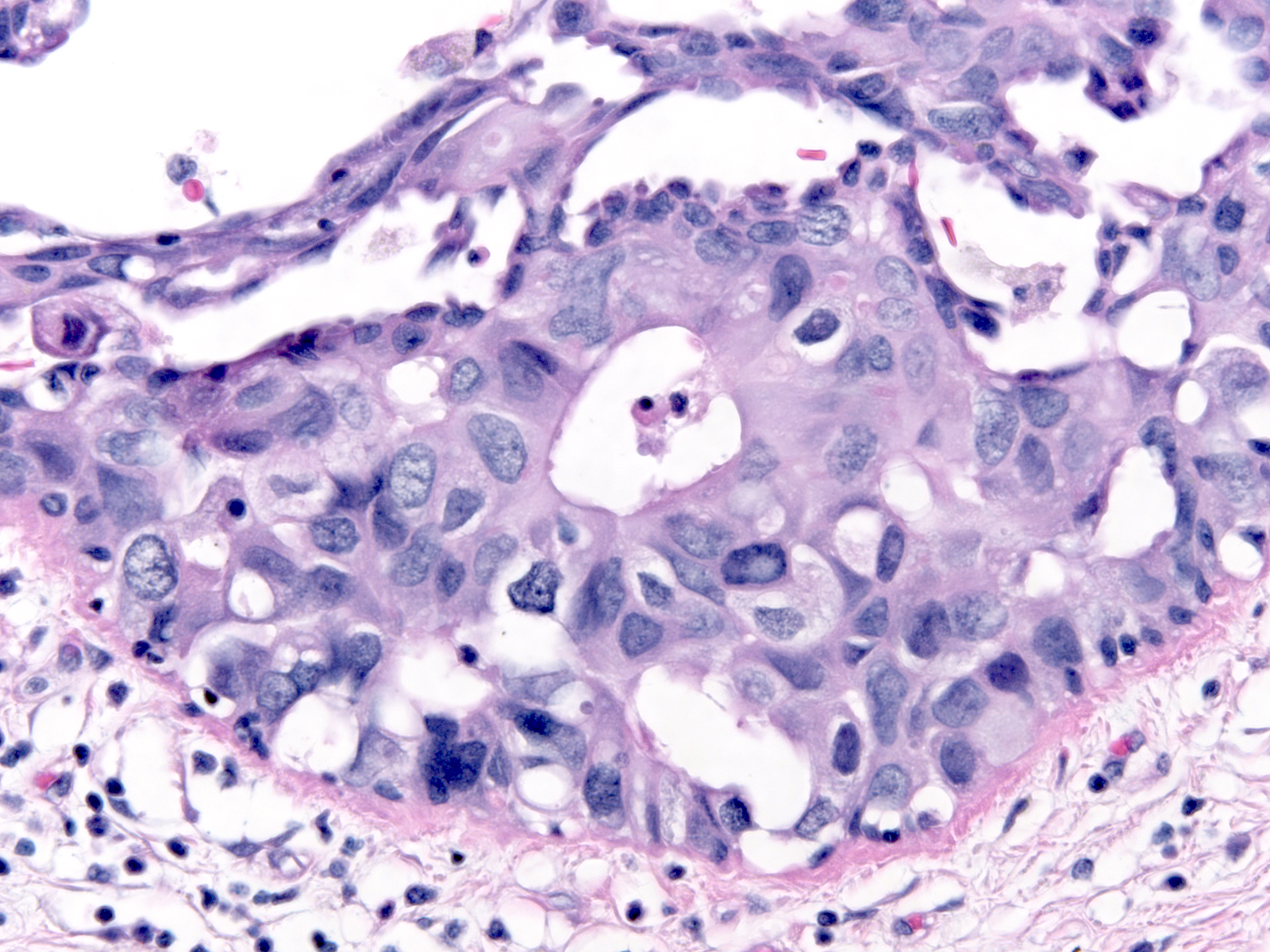
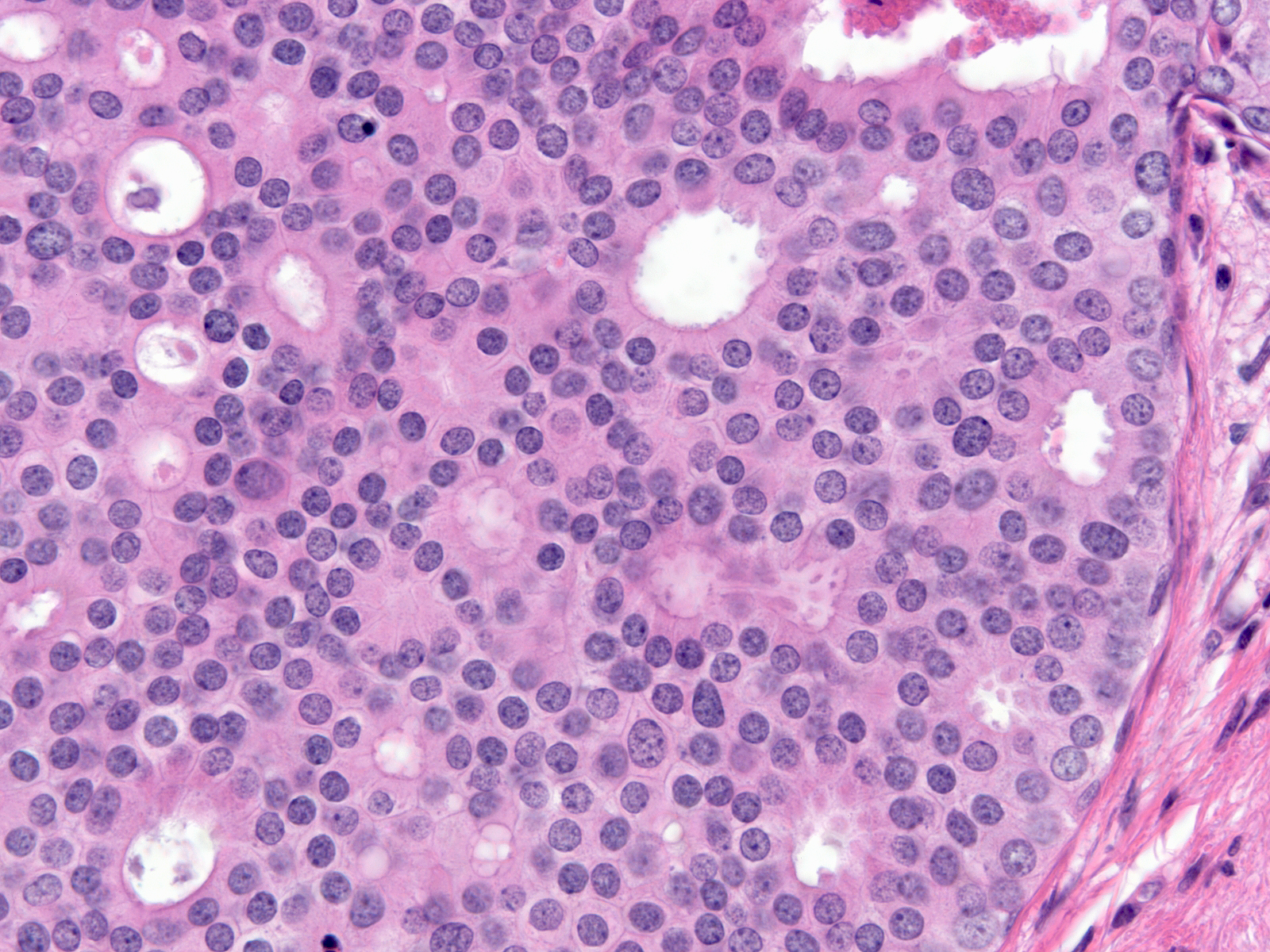
| Besides displaying polarization, the cells of DCIS demonstrate enlargement of the nucleus and increase in the amount of the cytoplasm; thus, the size of the cell increases. Contrasting the malignant cells with nearby benign cells highlights these alterations. In the focus to the right we can easily compare the size of the cells in an entrapped benign gland (center) to the size of the surrounding DCIS cells. |
 |
|
|
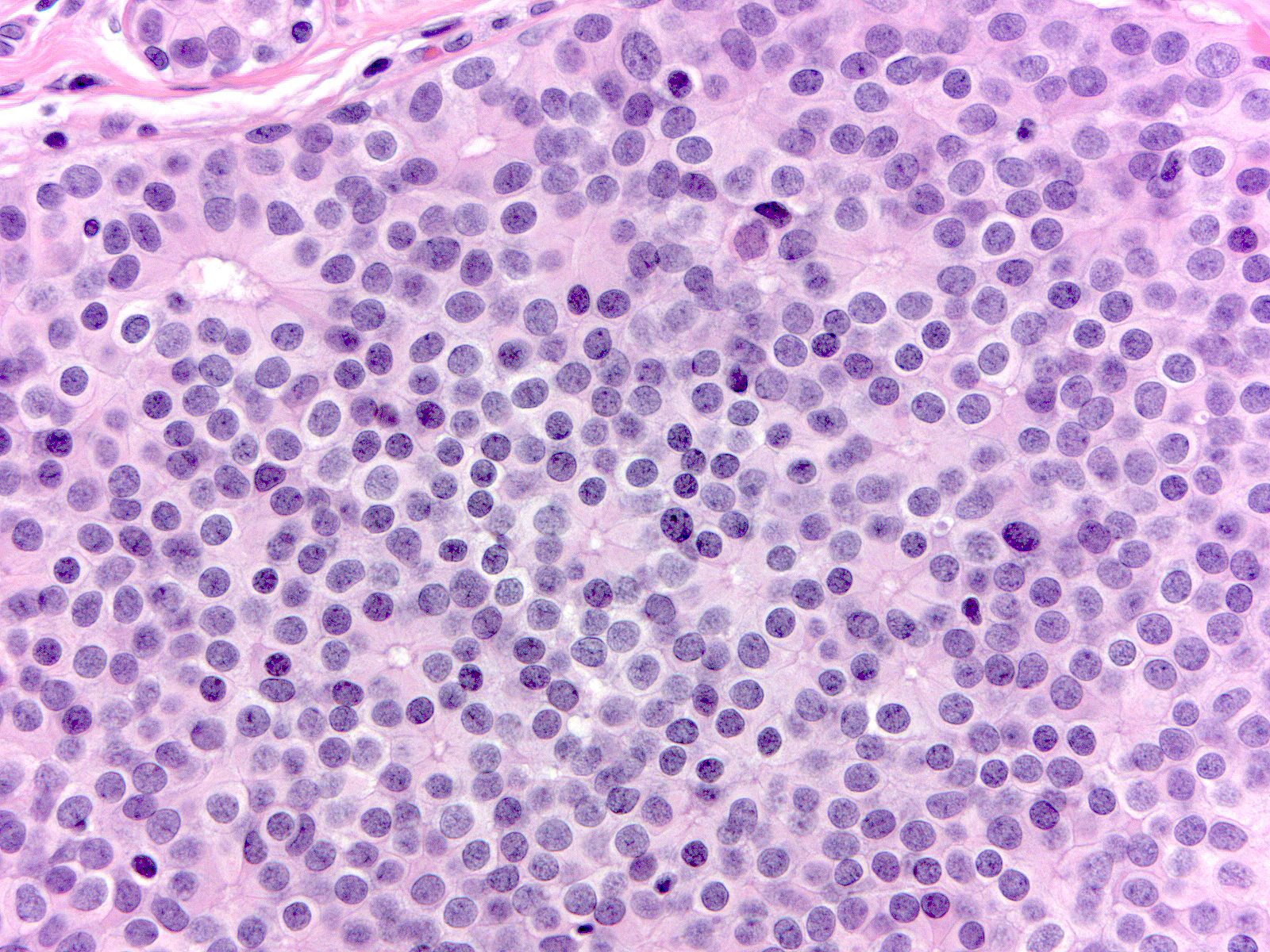
Cytologic Atypia
| The cytological atypia that characterizes DCIS varies according to the grade of the carcinoma. Features of low-grade atypia include: uniformity of the nuclei, round or oval nuclear shapes, smooth nuclear contours, and inconspicuous nucleoli. |
 |
|
|
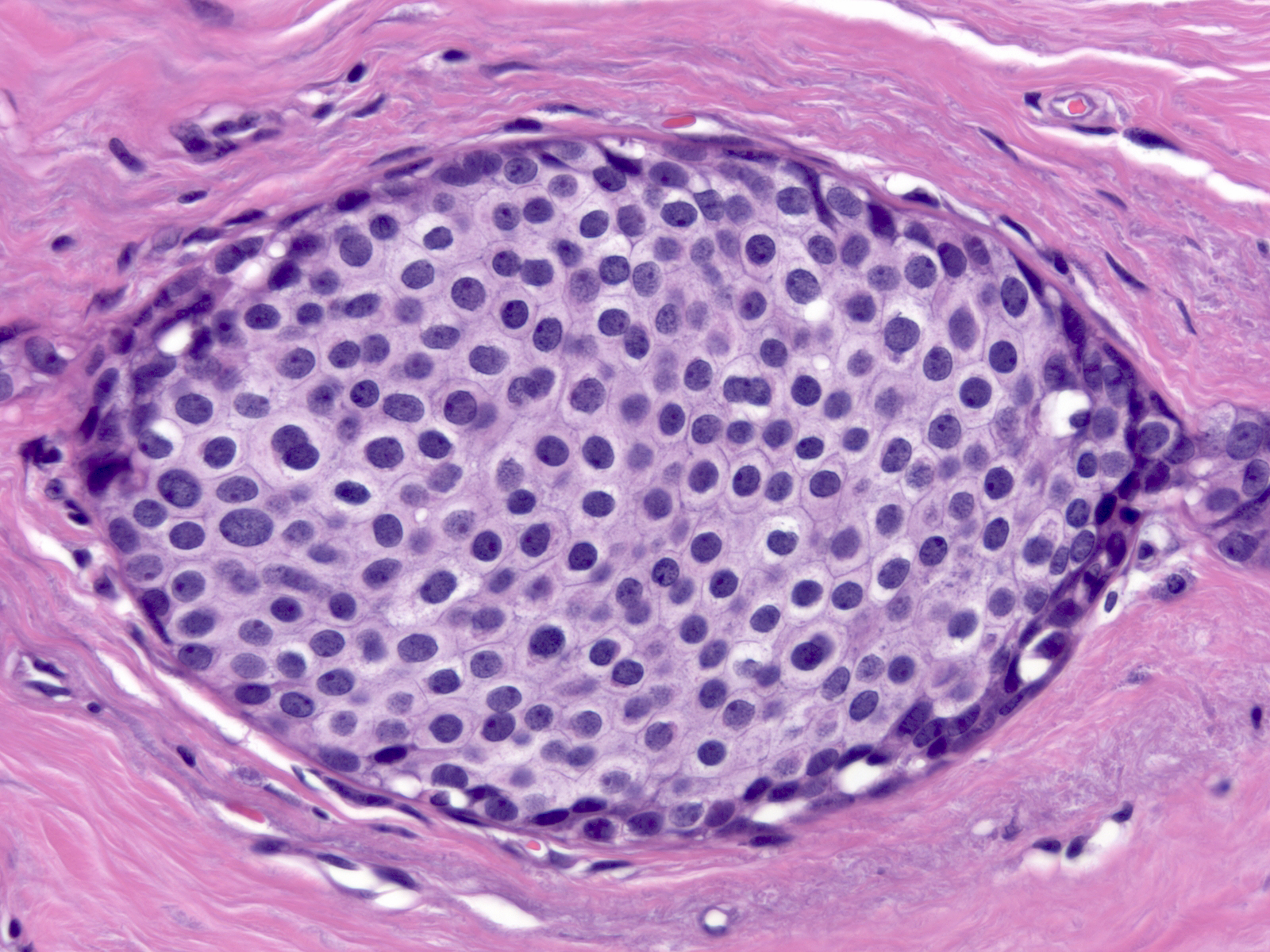
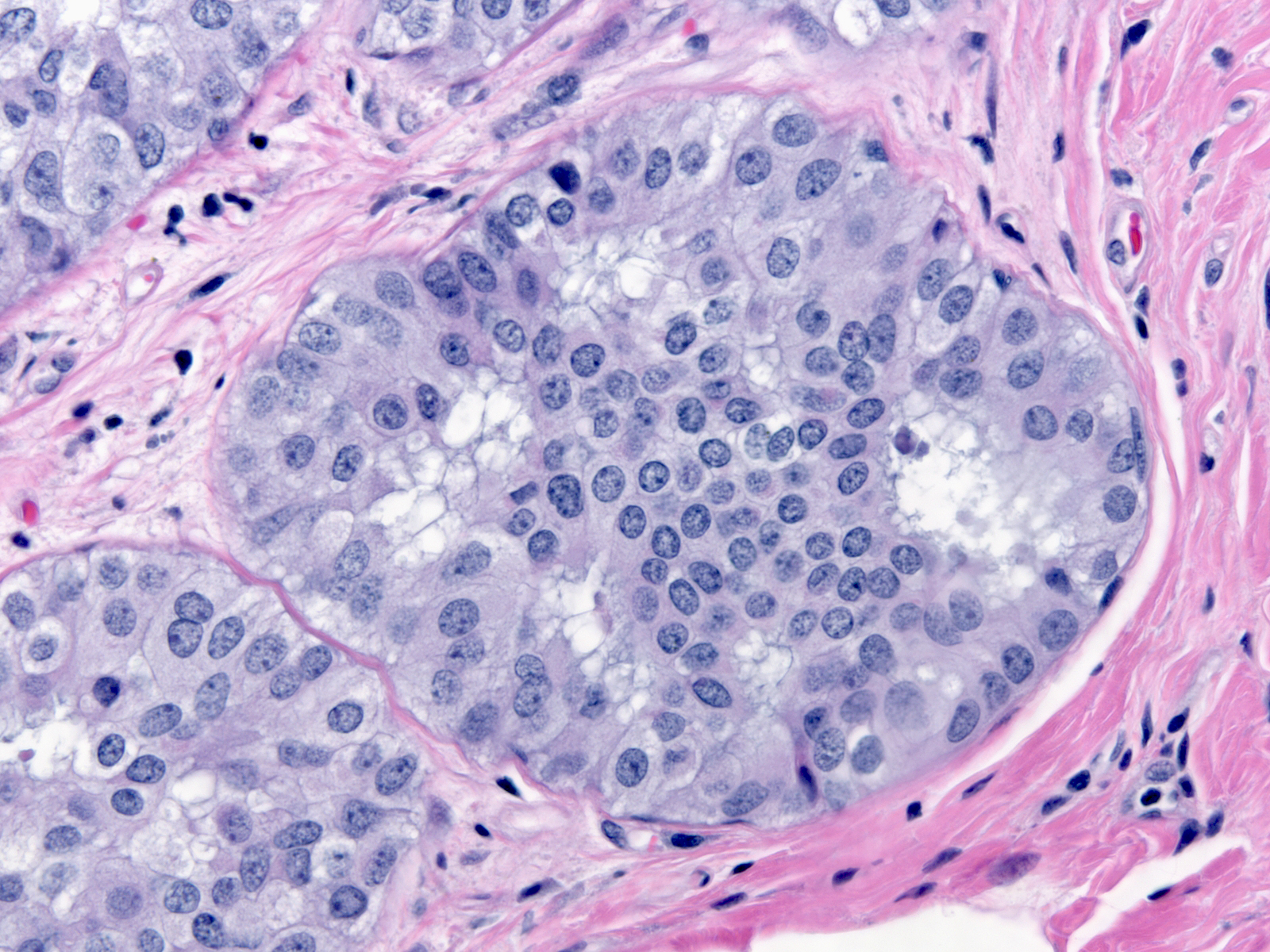
The chromatin of low-grade atypia can appear dark and dispersed (below left) or somewhat pale and finely granular (below middle). When it is pale and finely granular, you can usually see small nucleoli (below right).
 |
 |
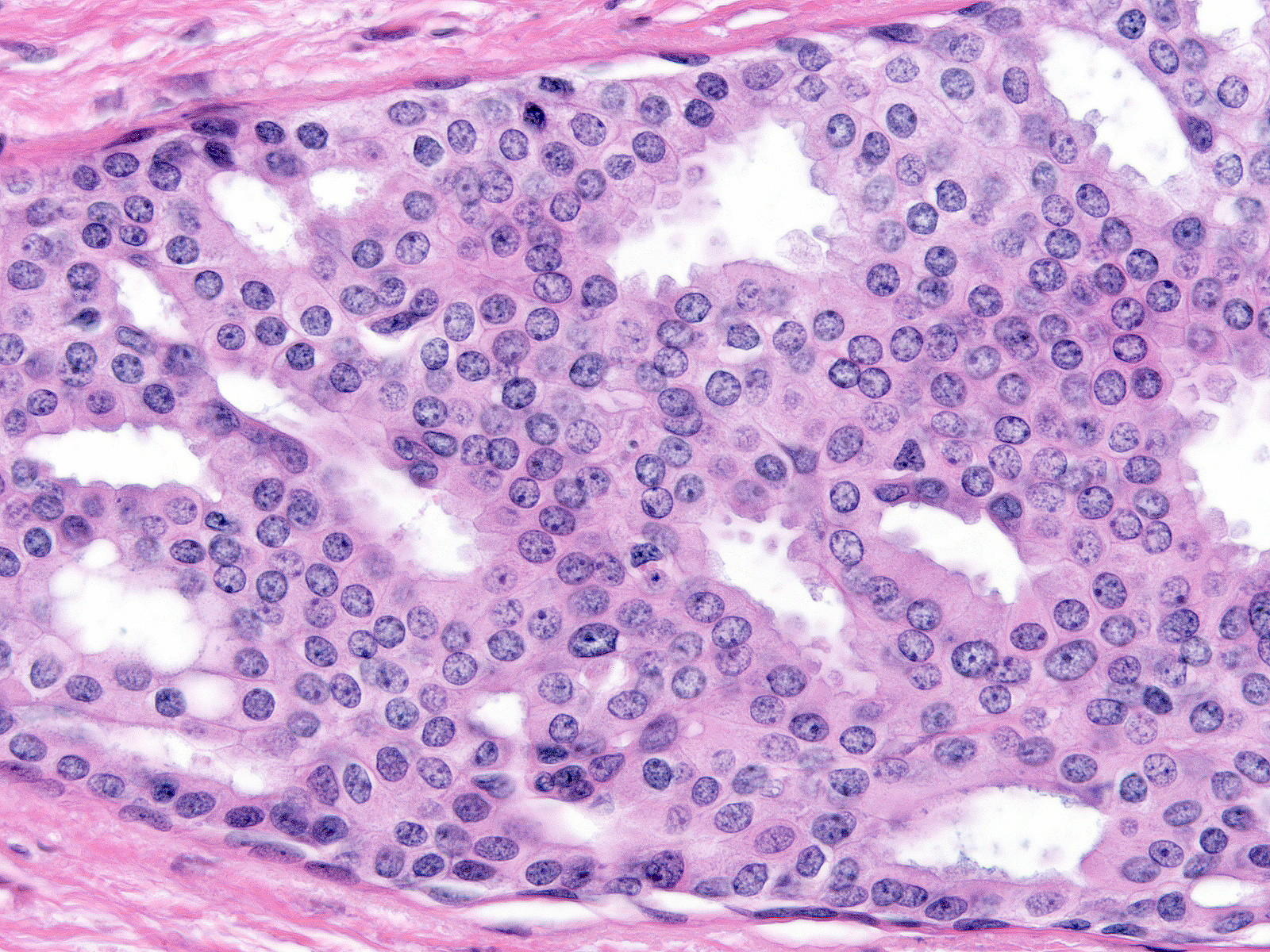 |
| Chromatin: dark and dispersed |
Chromatin: pale and finely granular |
Small nucleoli |
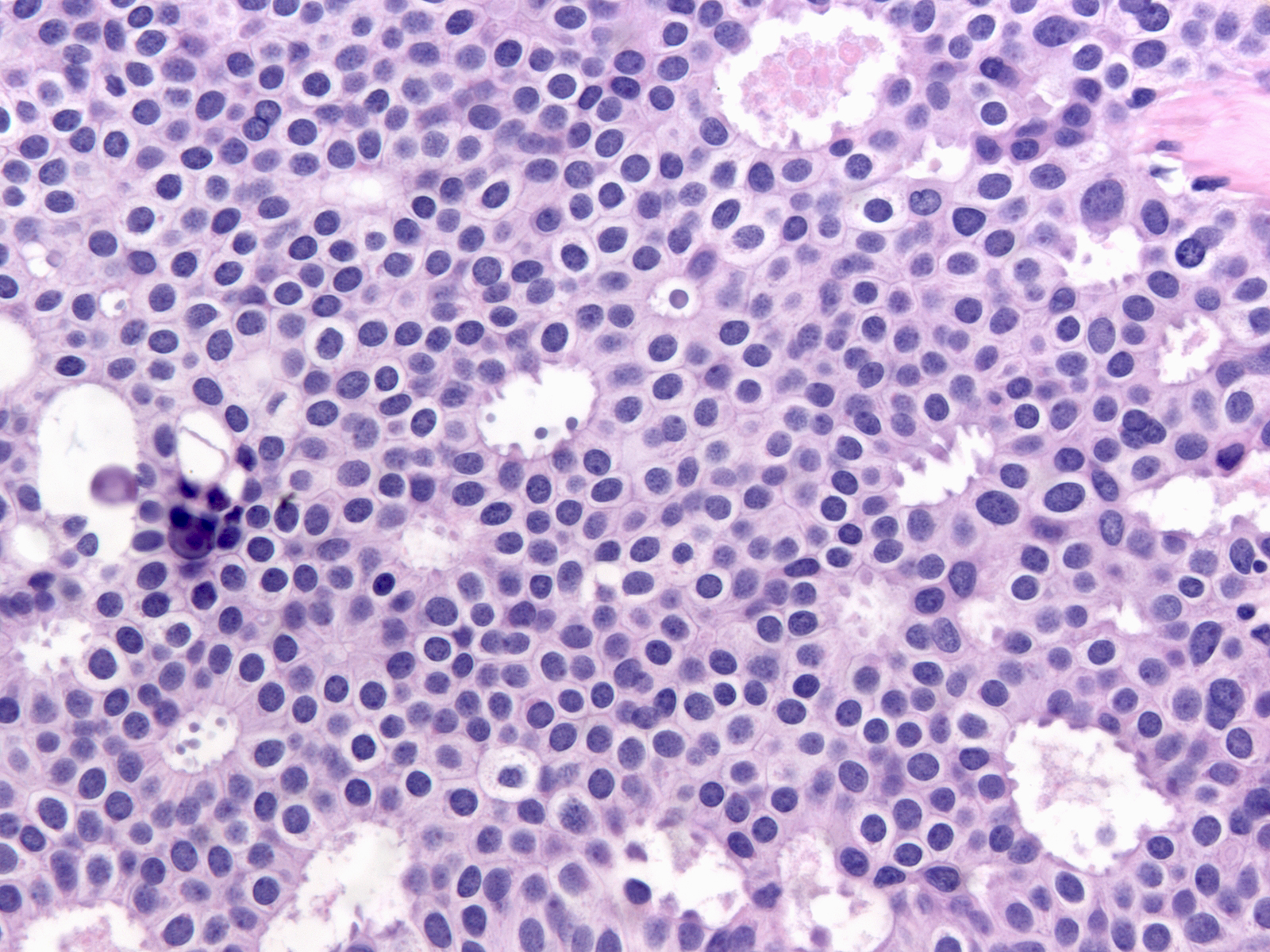
The cytoplasm of low-grade atypia usually appears pale, delicate, and faintly eosinophilic to amphophilic (below left), but certain examples consist of cells with clear (below middle) or densely eosinophilic (below right) cytoplasm.
 |
 |
 |
| Cytoplasm: faintly eosinophilic to amphophilic |
Cytoplasm: clear |
Cytoplasm: densely eosinophilic |
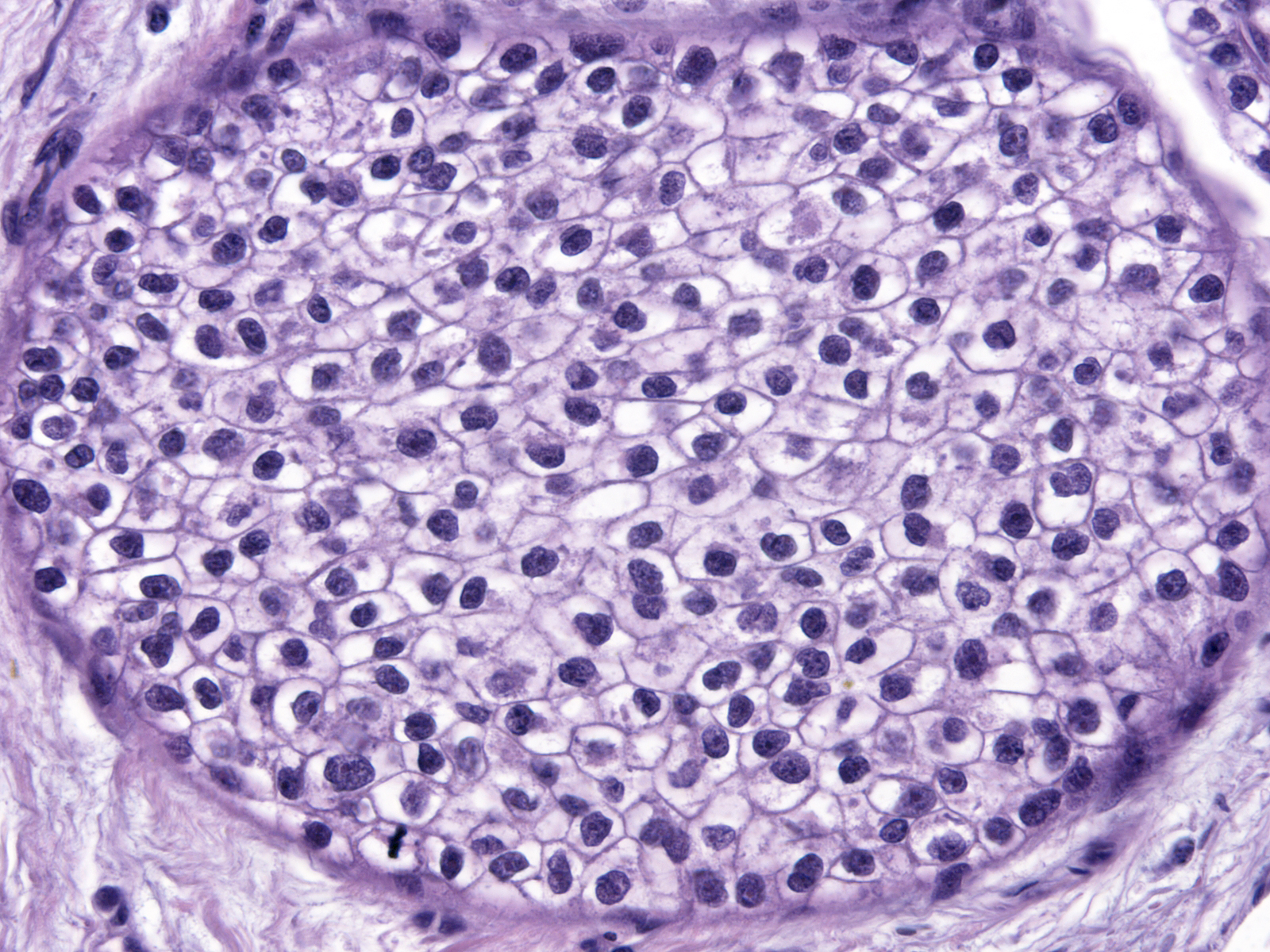
| In low-grade atypia, the cell borders often appear distinct, as they do in the picture to the right and the pictures above. |
 |
|
|
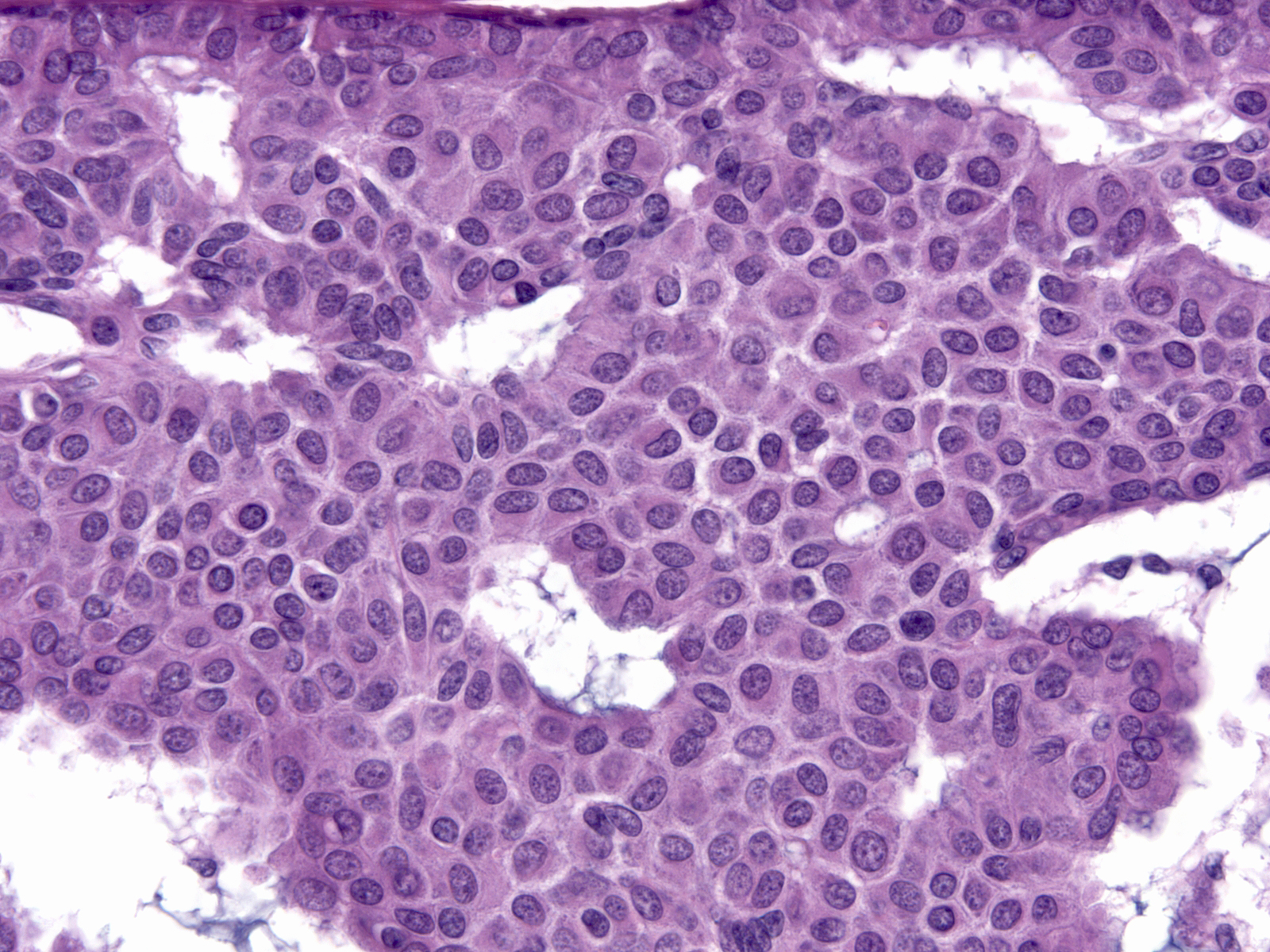
| In high-grade DCIS, cells appear greatly enlarged and they demonstrate obvious nuclear enlargement, variation in the size and shape of the nuclei, and variation in the number, size, and shape of the nucleoli. Mitotic figures abound, and the cells often undergo necrosis. |
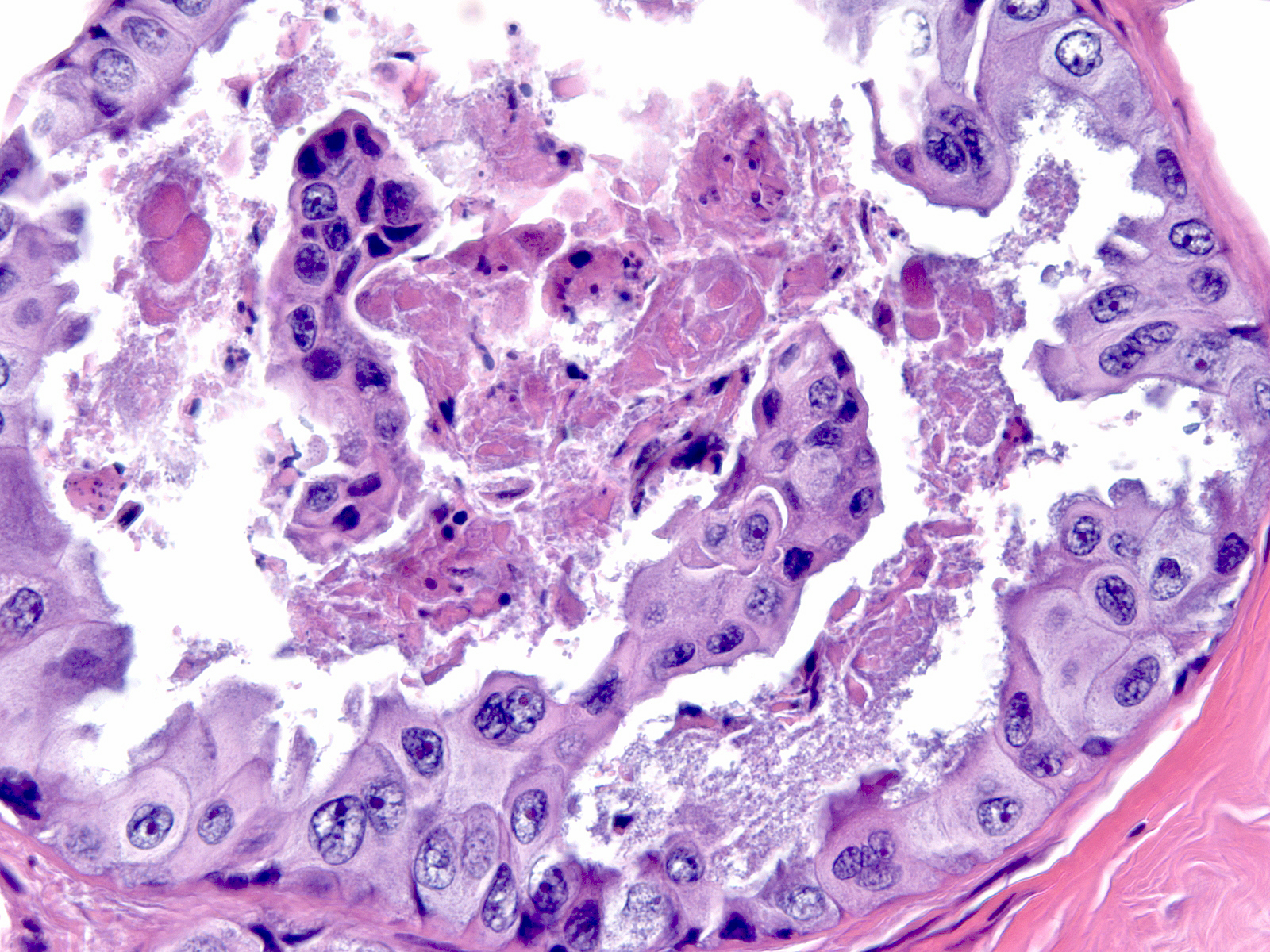 |
|
|
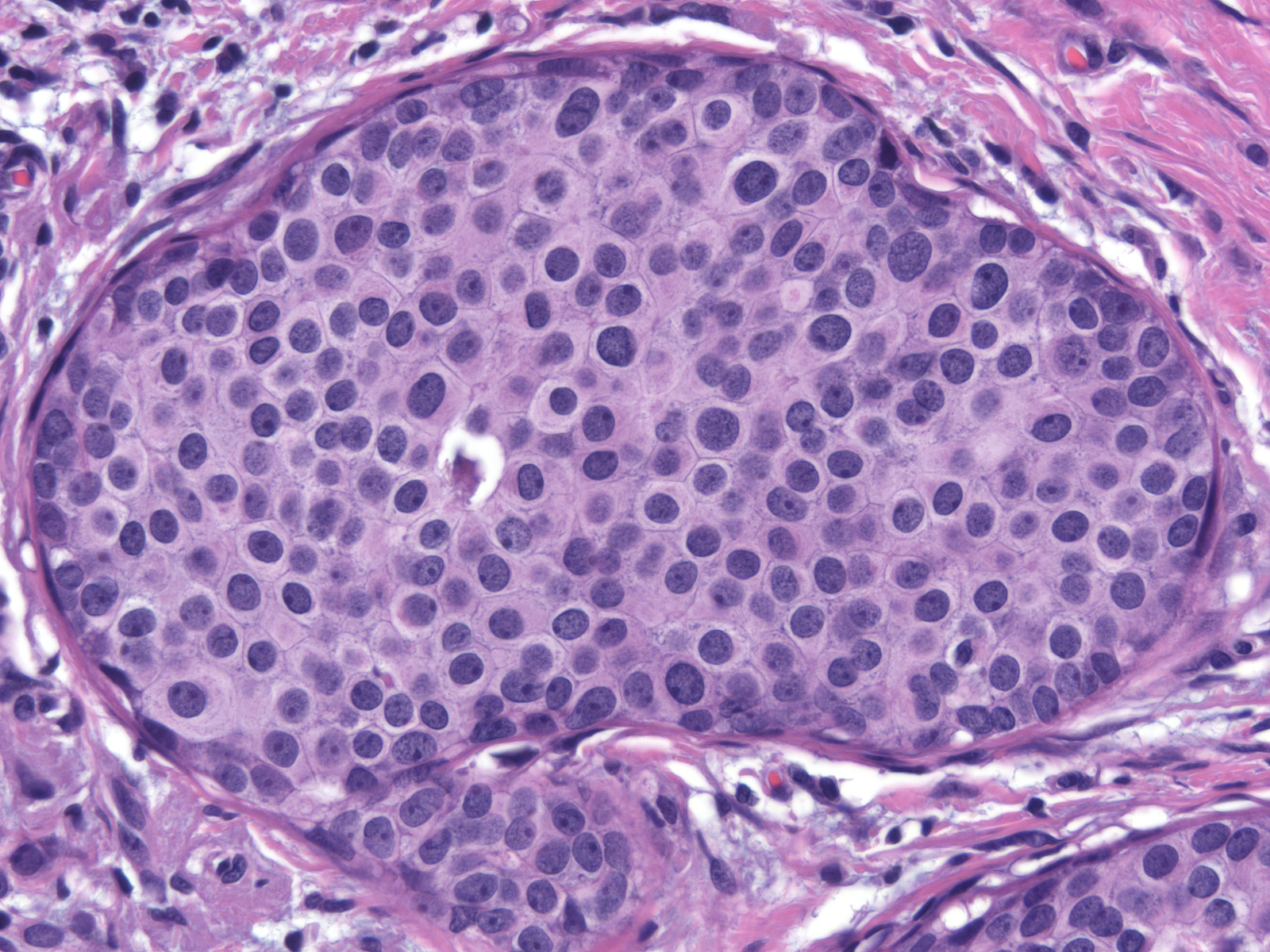
| Cases of intermediate-grade DCIS typically exhibit enlargement of the cells and their nuclei, but otherwise do not have a consistent set of cellular alterations. As in the case to the right, their nuclei and nucleoli appear more variable in size and shape than those of low-grade ductal carcinoma in-situ but less so than those of high-grade ductal carcinoma in-situ. |
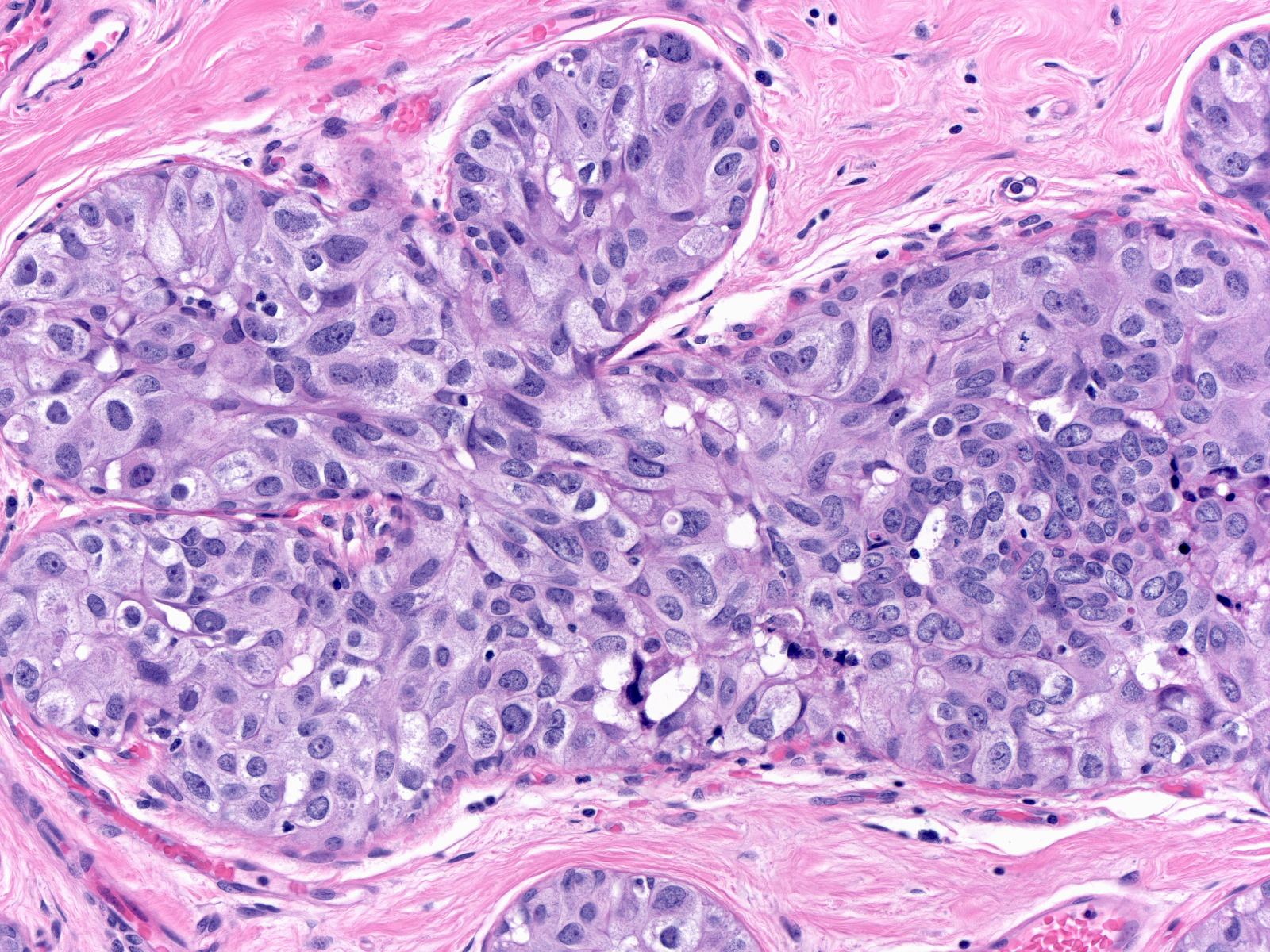 |
|
|
The chromatin of intermediate-grade DCIS varies from dark and smudgy (left) to pale and coarsely granular (right).
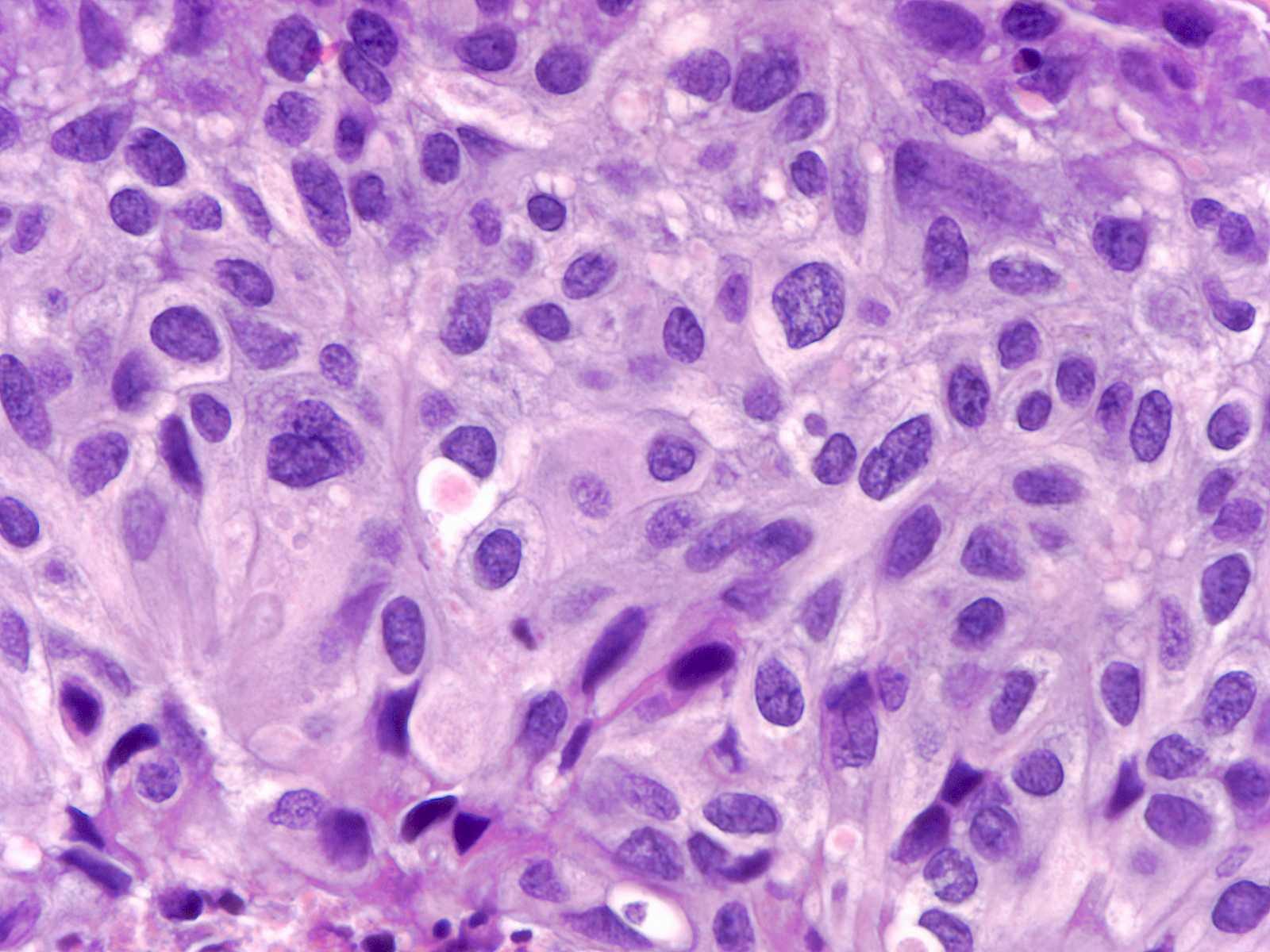 |
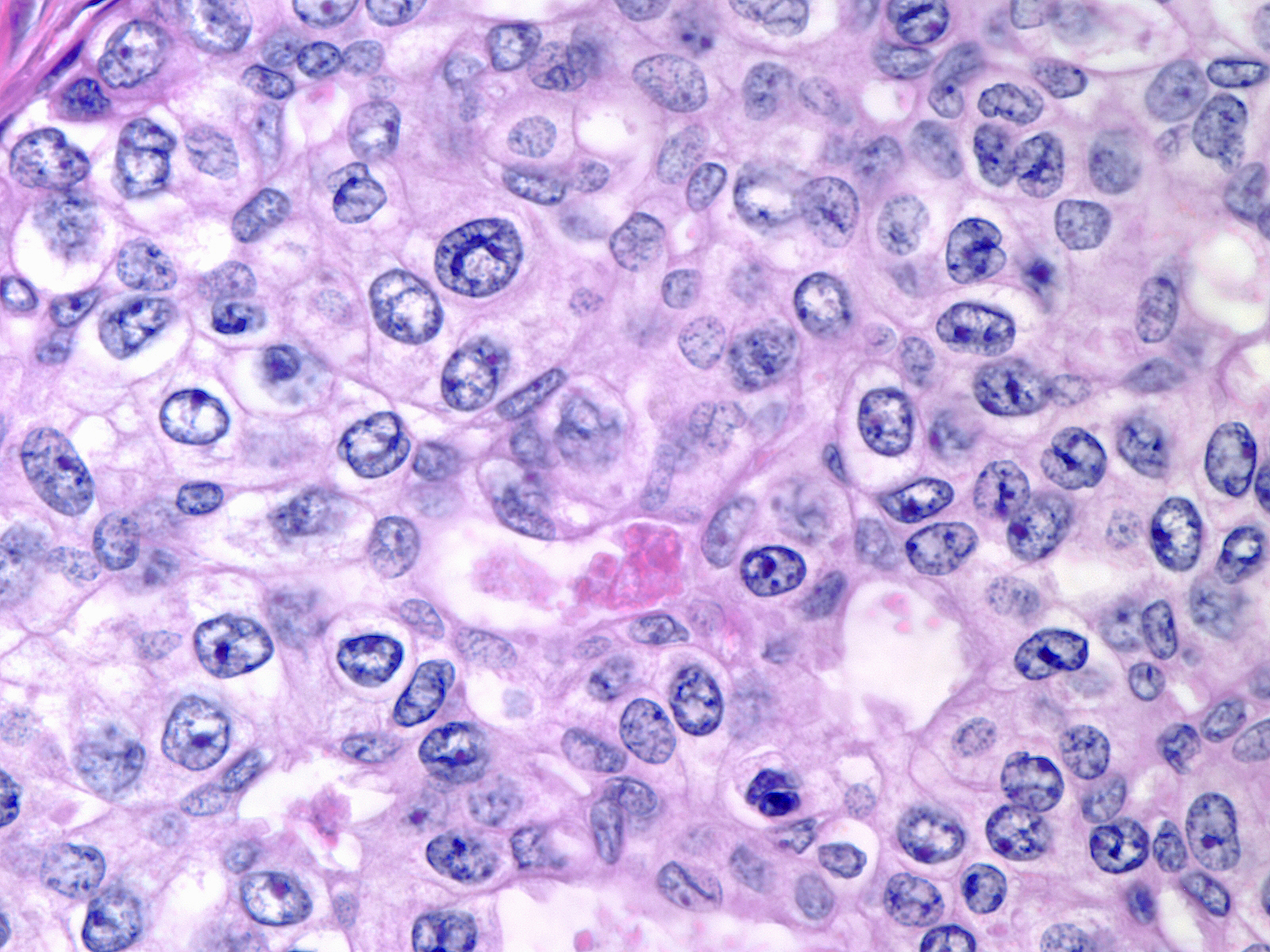 |
| Chromatin: dark and smudgy |
Chromatin: pale and coarsely granular |
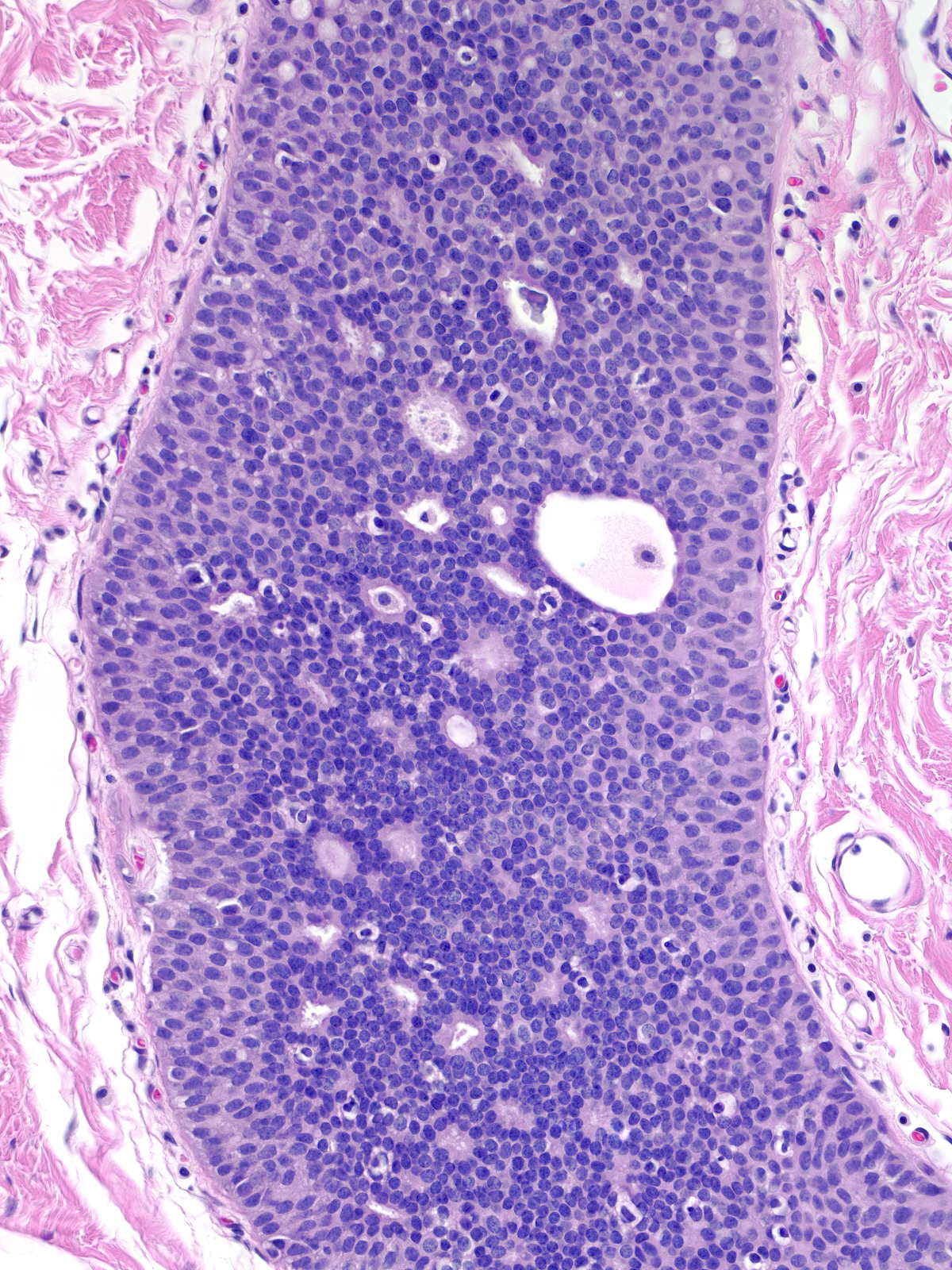
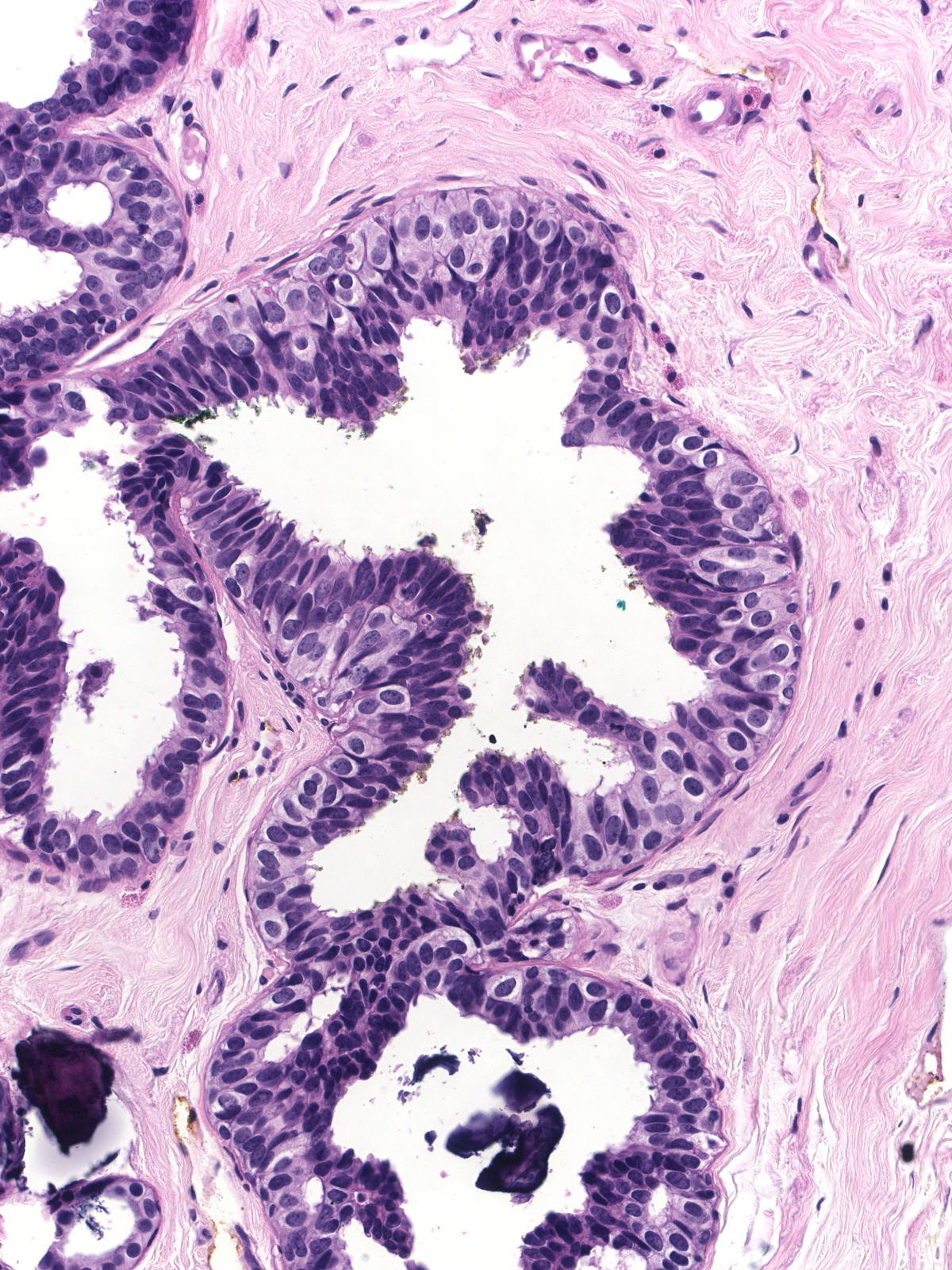
The variation in nuclei shape and the presence of nucleoli make it difficult to distinguish intermediate-grade ductal carcinoma in-situ (below left) from usual ductal hyperplasia (below right). When present, clearly defined cell membrane, uniform chromatin, or uniform nucleoli would favor the diagnosis of intermediate‑grade ductal carcinoma in-situ, but many cases lack these findings. The enlargement of the cells and their nuclei represents the most commonly encountered and most reliable finding that distinguishes intermediate-grade ductal carcinoma in-situ from usual ductal hyperplasia. Contrast the cell and nuclear sizes, the cell membranes, and the uniformity of the chromatin and nucleoli. Neoplastic ductal cells also appear somewhat dishesive compared to the marked cohesion characteristic of hyperplastic ductal cells.
 |
 |
| Intermediate-grade ductal carcinoma in-situ |
Usual ductal hyperplasia |
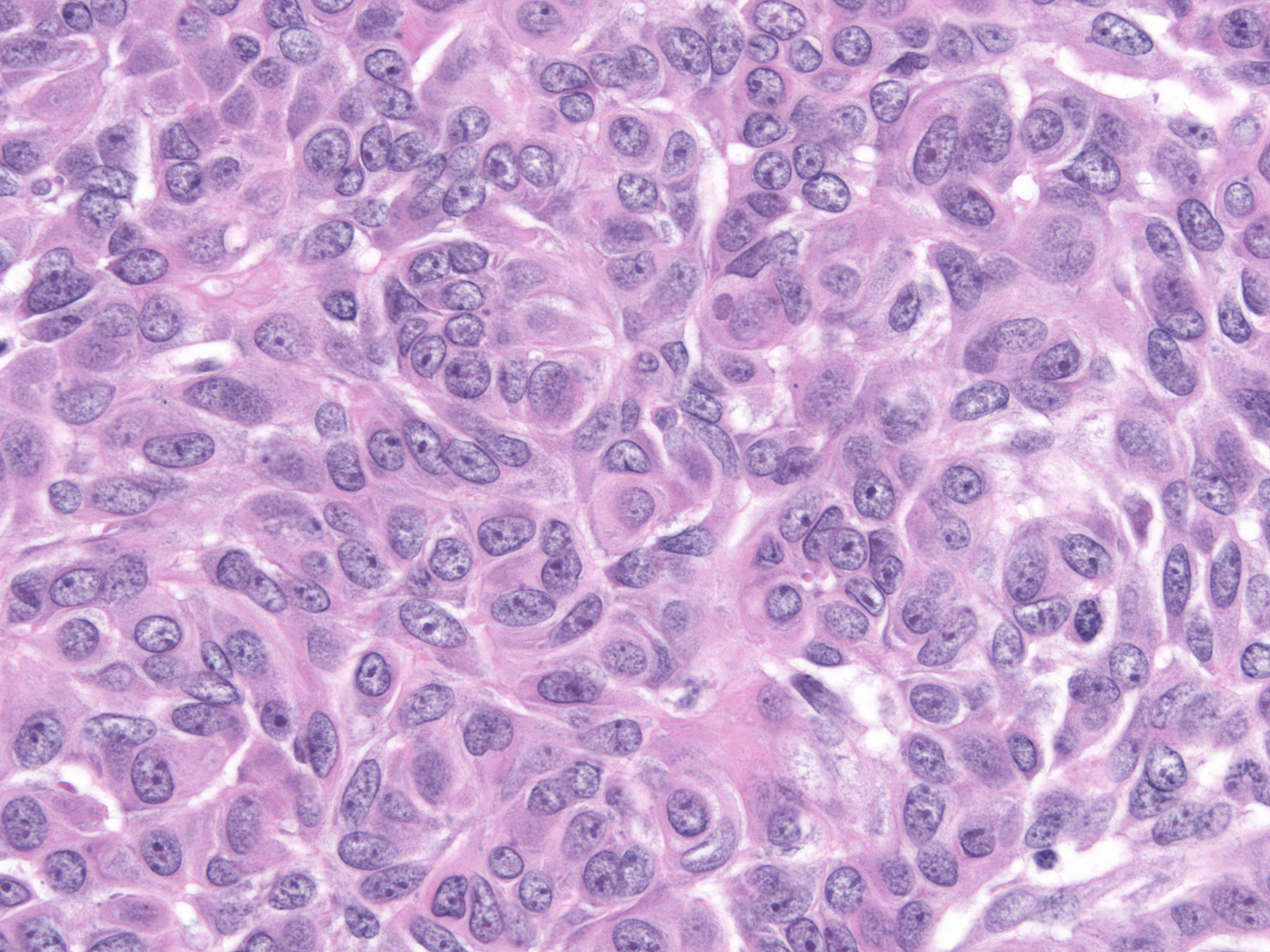
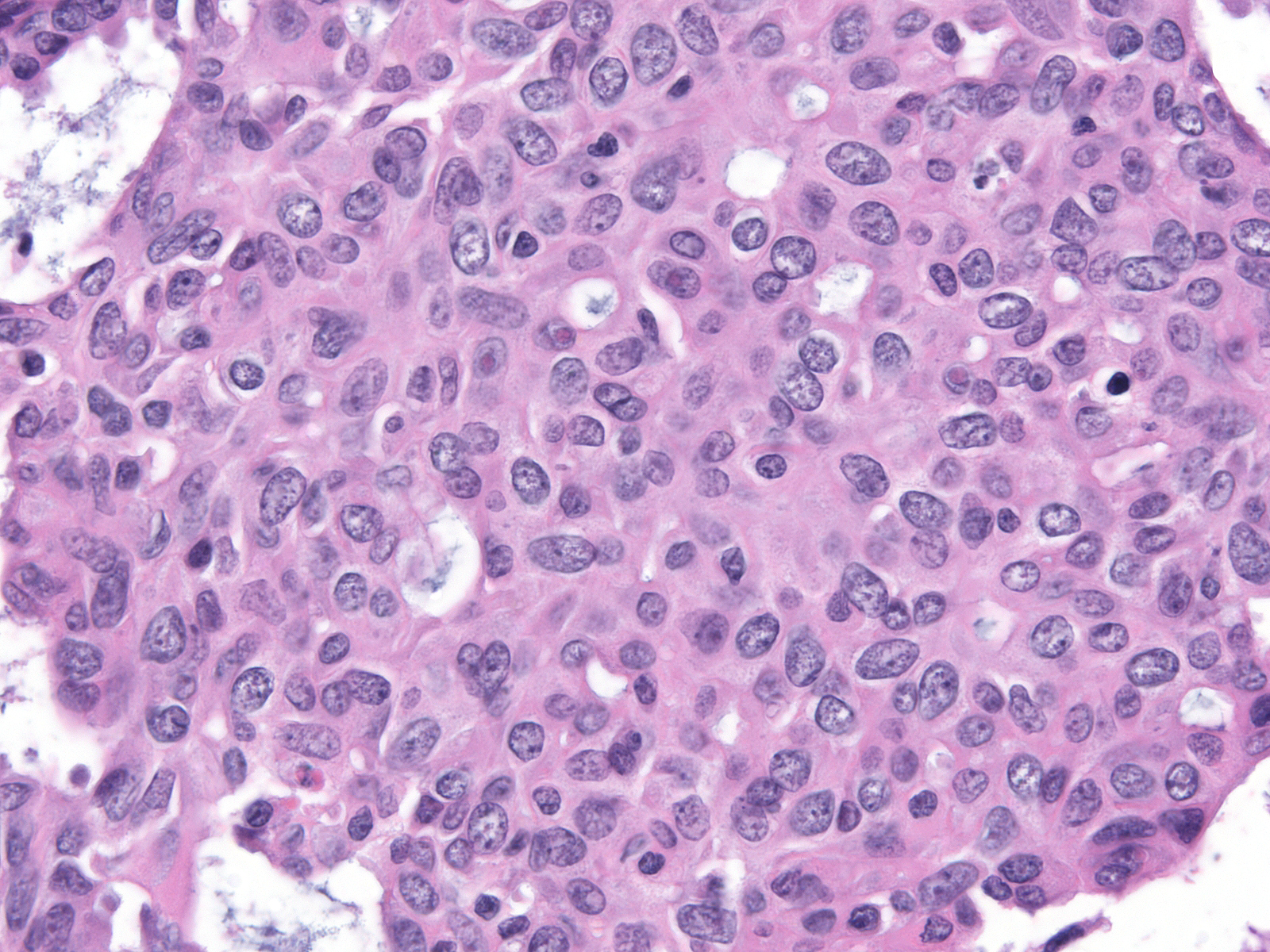
Cases of ductal carcinoma in‑situ sometimes exhibit regional variation in the cytological attributes of their cells. Pathologists refer to one repetitive pattern of variation as dimorphism. In this phenomenon, the neoplastic cells along the basement membrane appear larger than the central cells. The peripheral cells have more cytoplasm, which appears pale, larger nuclei, and, often, larger nucleoli compared to the central cells.
 |
 |
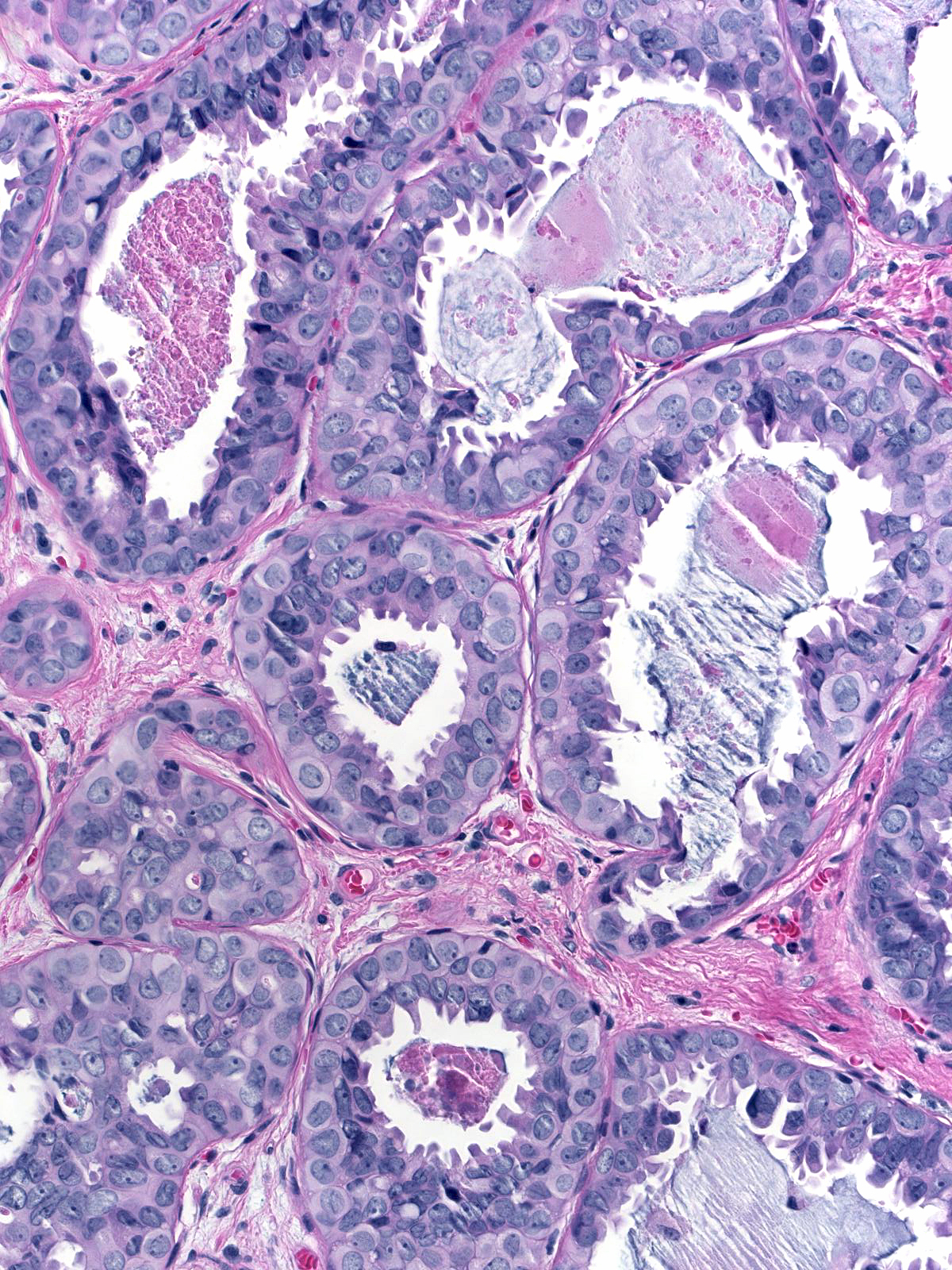 |
| Low-grade dimorphic carcinoma |
Intermediate-grade dimorphic carcinoma |
High-grade dimorphic carcinoma |
The pale cytoplasm brings to mind the appearance of active myoepithelial cells. Staining for markers of myoepithelial cells will help one to avoid this misinterpretation. The phenomenon of maturation represents a more likely source of confusion.
| Attention to the nuclear features of the central cells will usually clarify the nature of the proliferation. The nuclei of the central cells in a focus of ductal hyperplasia with maturation (left) do not appear atypical; instead, they display the features of hyperplastic nuclei. The central cells in an example of dimorphic ductal carcinoma in‑situ, on the other hand, demonstrate atypia (right). |
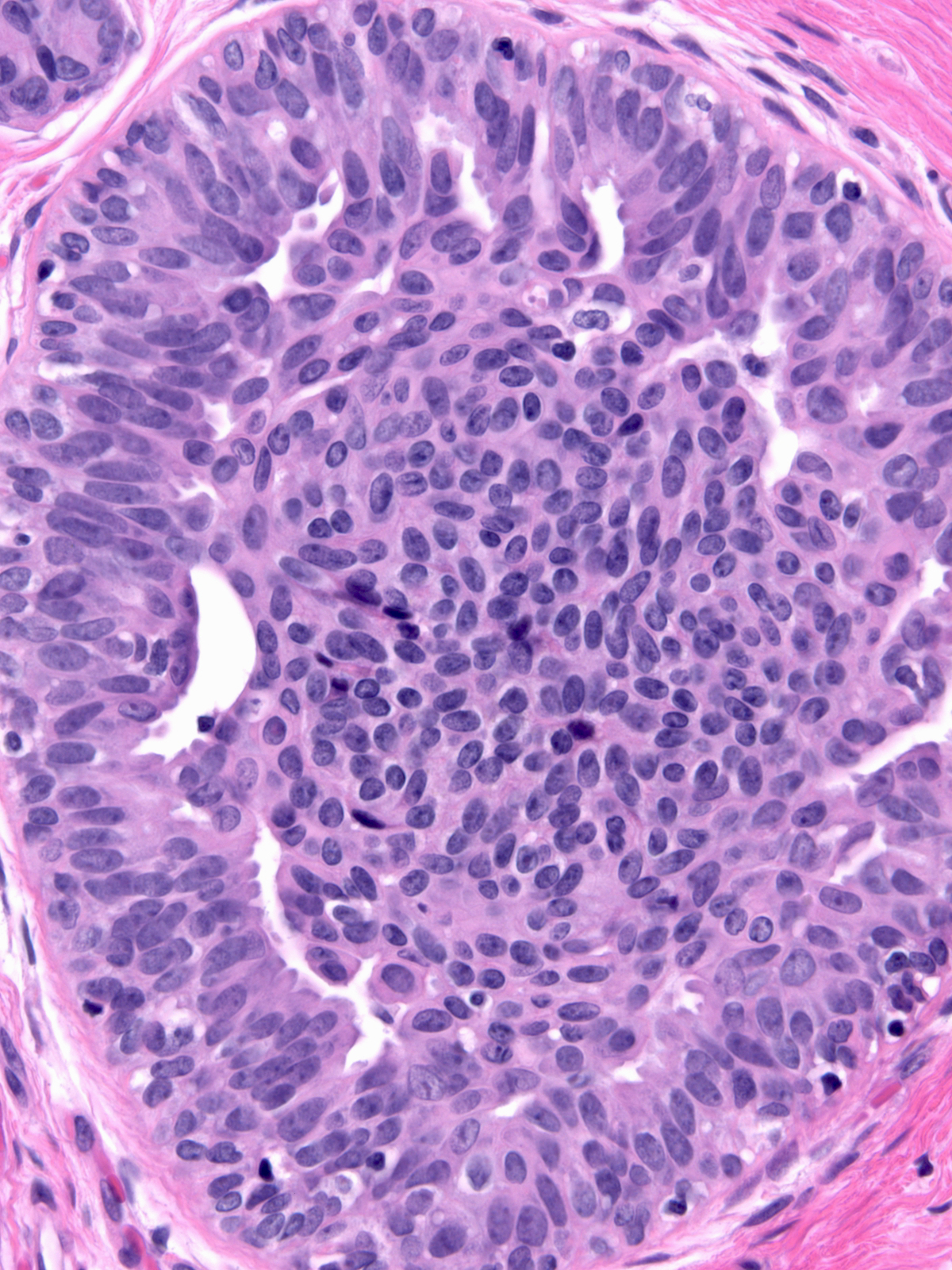 |
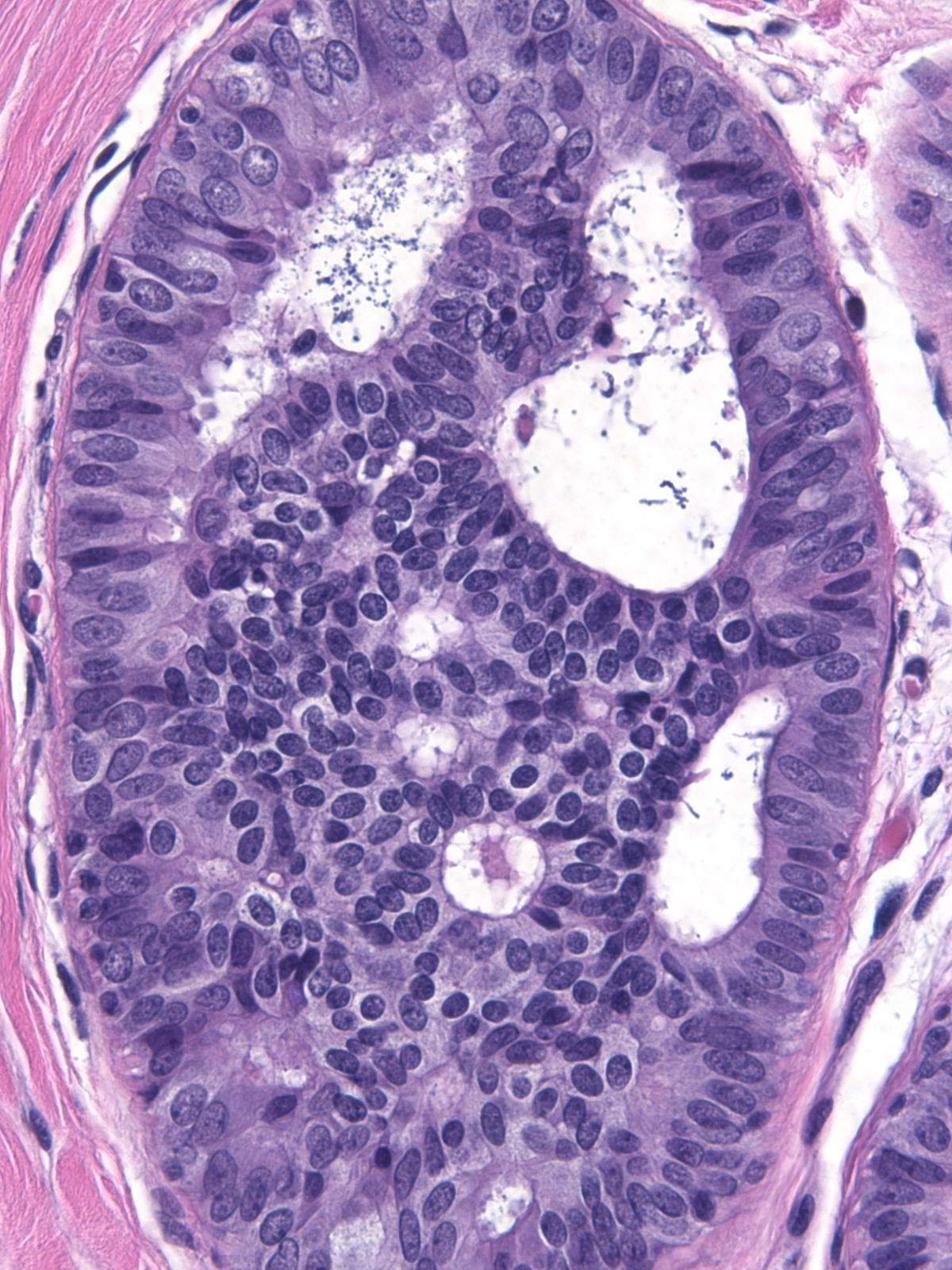 |
|
|
|
Architectural Atypia
Ductal carcinoma in-situ grows in several architectural patterns: cribriform, micropapillary, solid, and comedo. Many cases show a mixture of growth patterns.
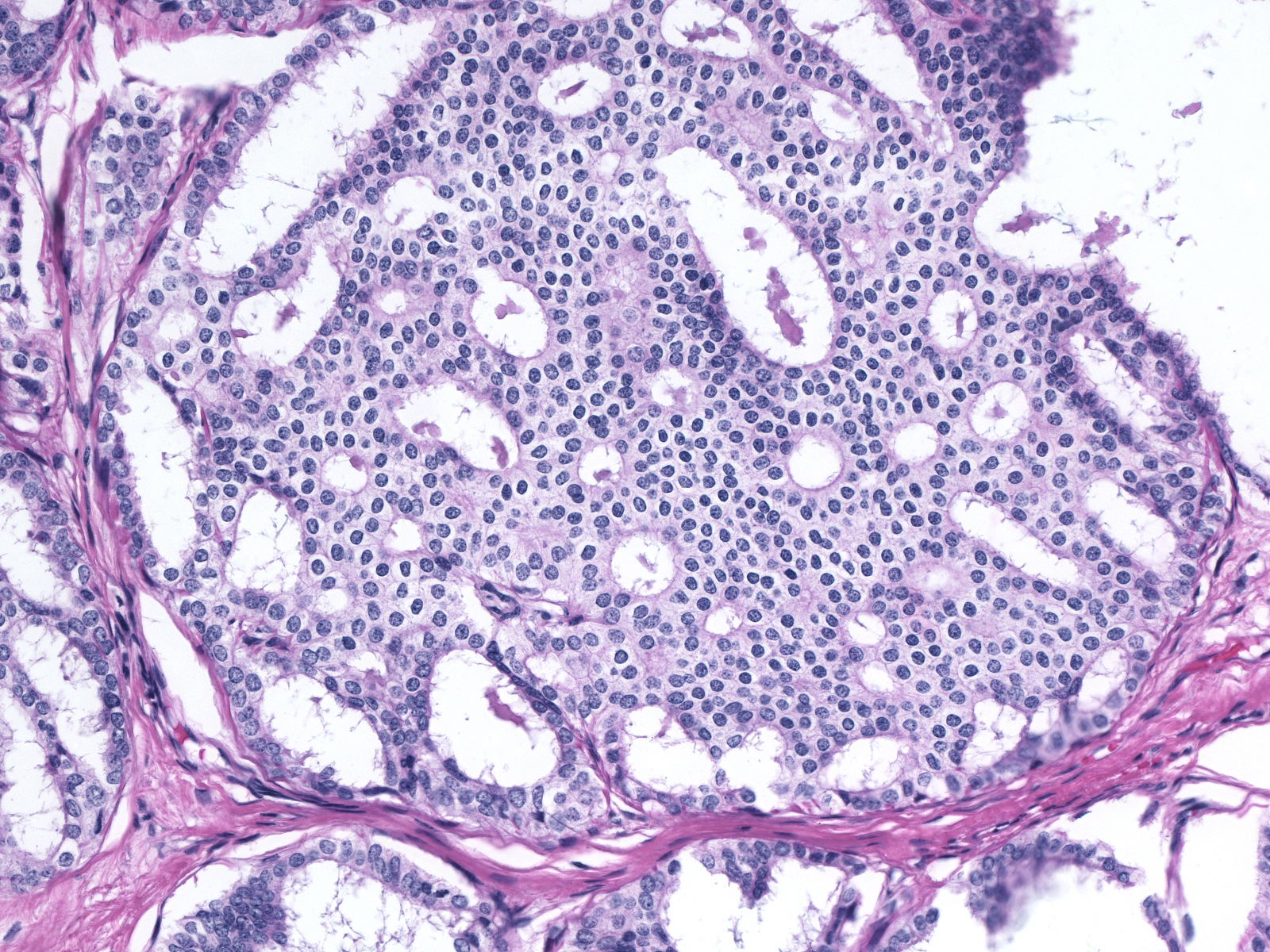 |
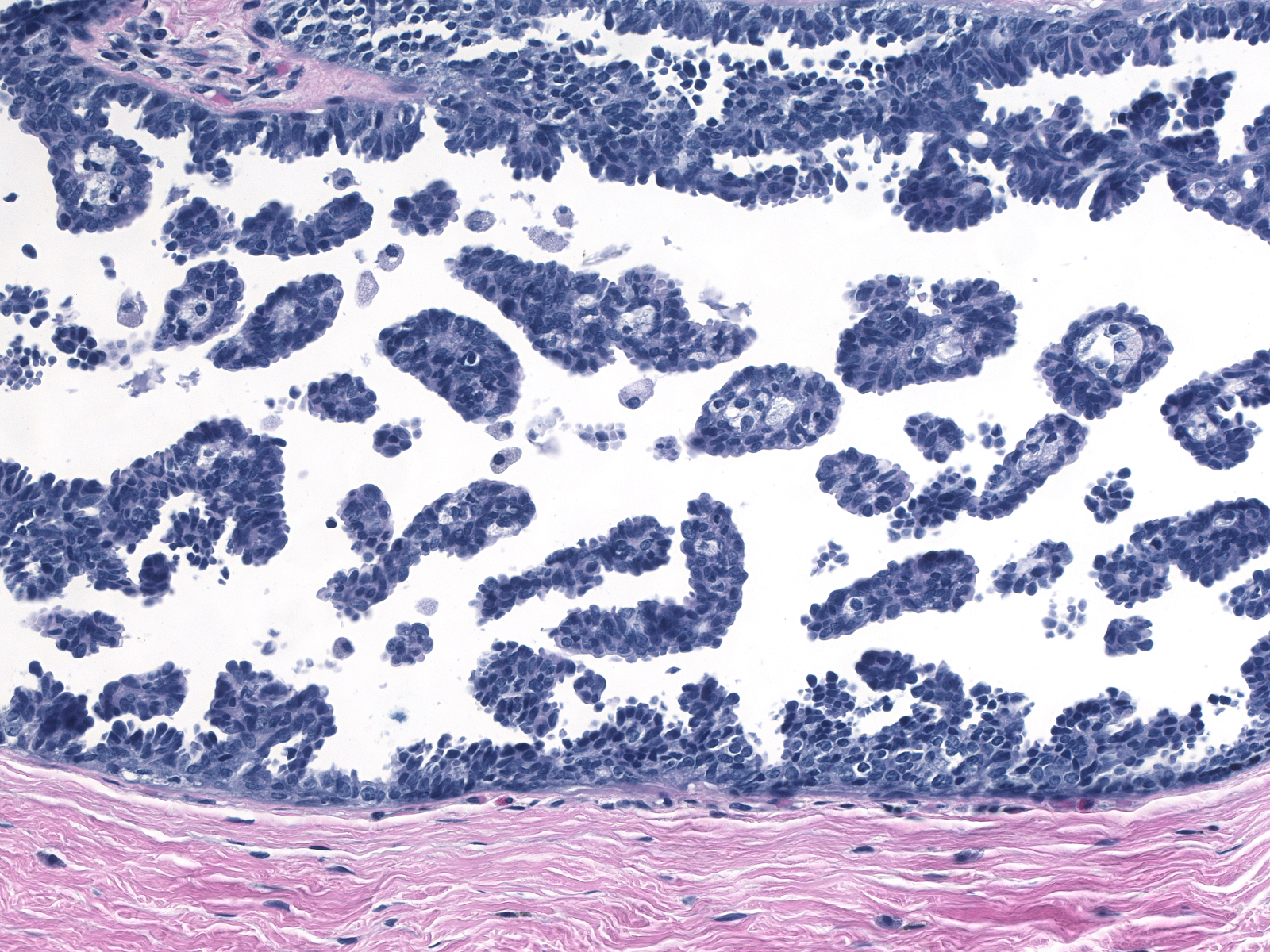 |
| Cribriform |
Micropapillary |
 |
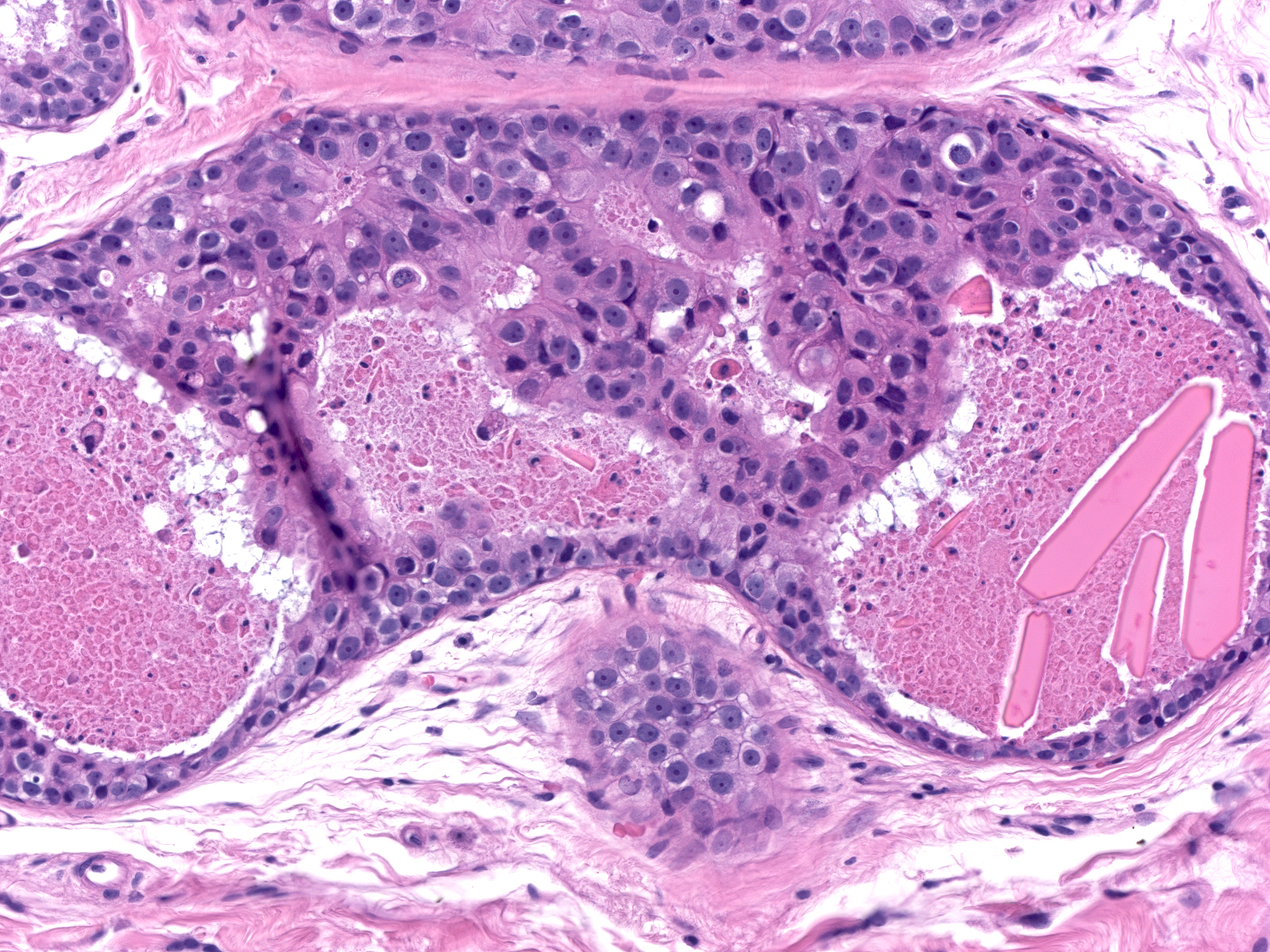 |
| Solid |
Comedo |
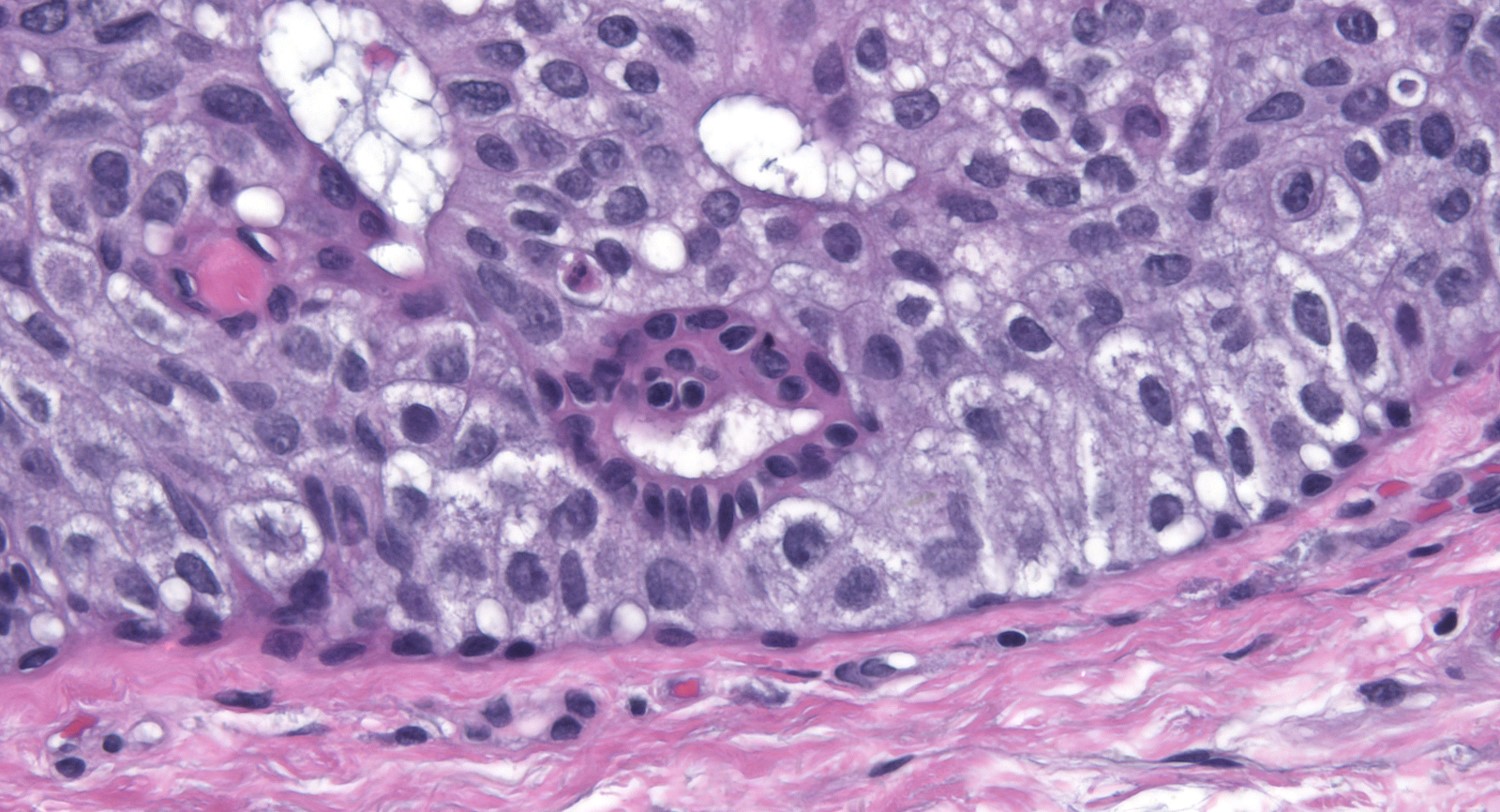
| One recognizes cribriform spaces by the polarized nature of the cells creating the space. |
 |
|
|
| The space need not appear round, but the enclosing cells must appear polarized. |
 |
|
|
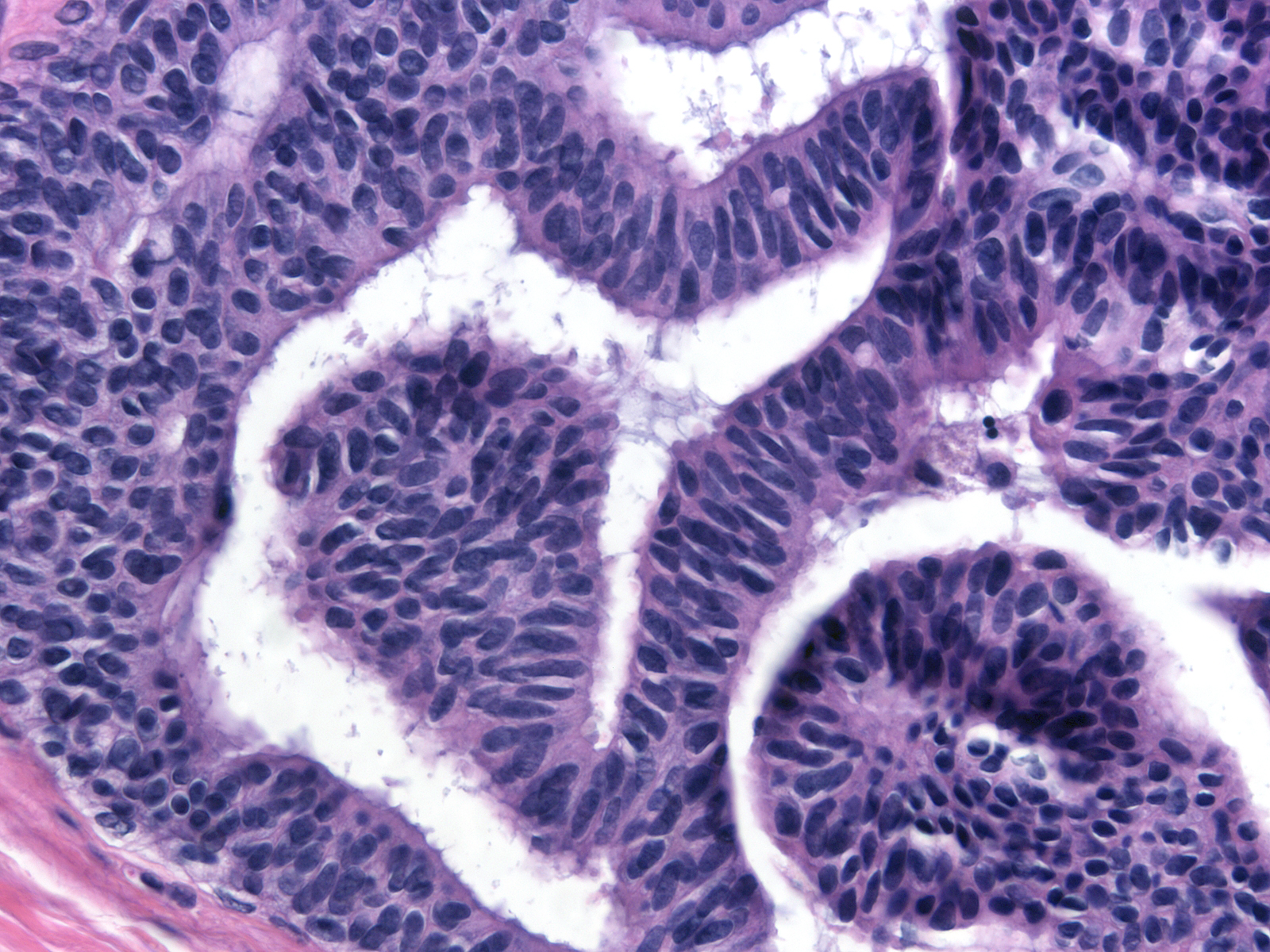
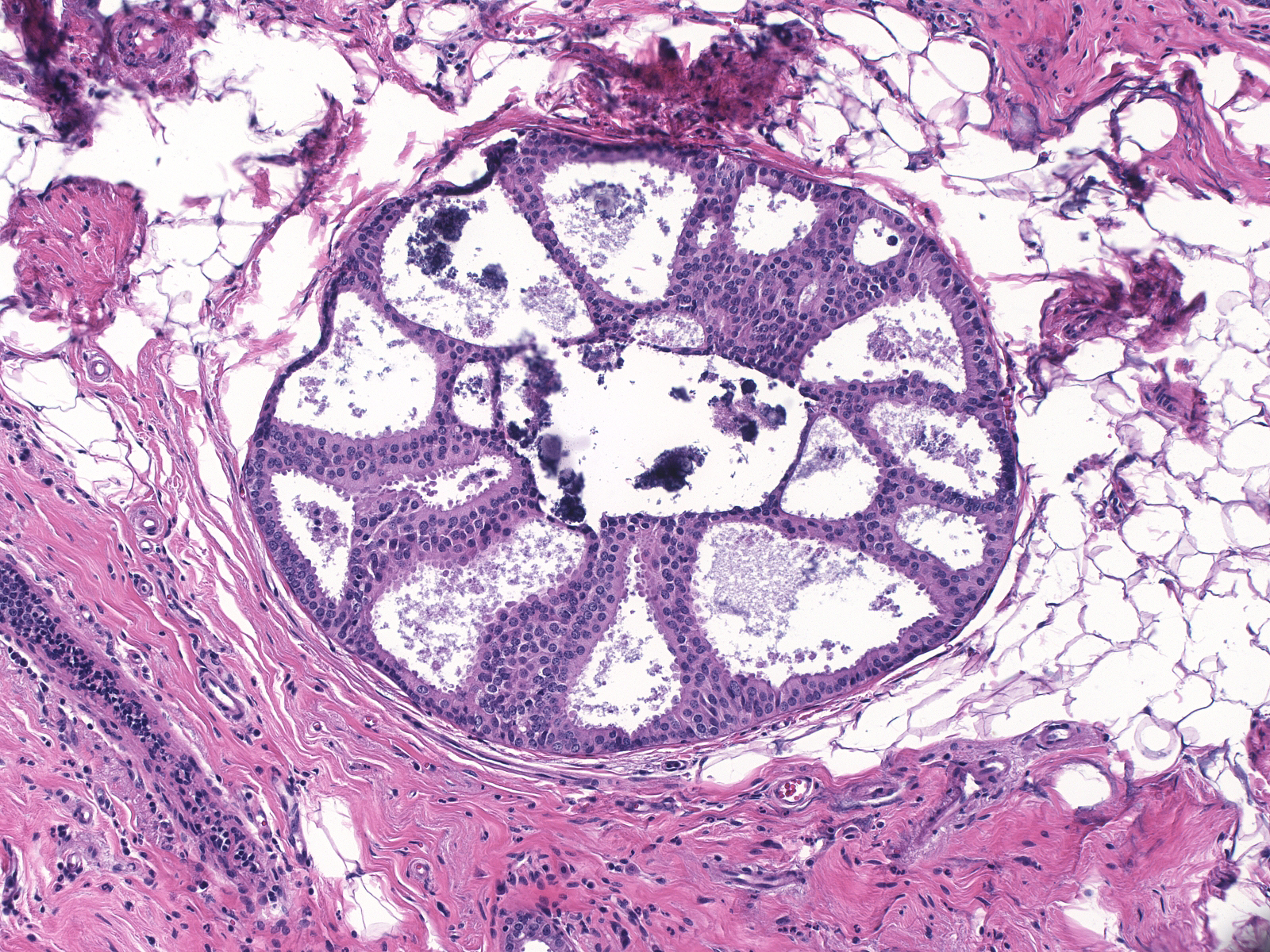
Several architectural patterns represent variants of cribriform spaces: trabecular bars, cartwheels, and Roman bridges. A trabecular bar is a column of proliferative cells in which the long axes of the cells lie perpendicular to the long axis of the column. A cartwheel formation consists of a group of radially arranged trabecular bars resembling the spokes of a wheel. A Roman bridge is an arching trabecular bar located at the periphery of a duct. The nuclei of the cells forming the bar appear radially oriented around the space enclosed by the bridge. These patterns constitute examples of architectural atypia.
 |
 |
 |
| Trabecular bar |
Cartwheel |
Roman bridge |
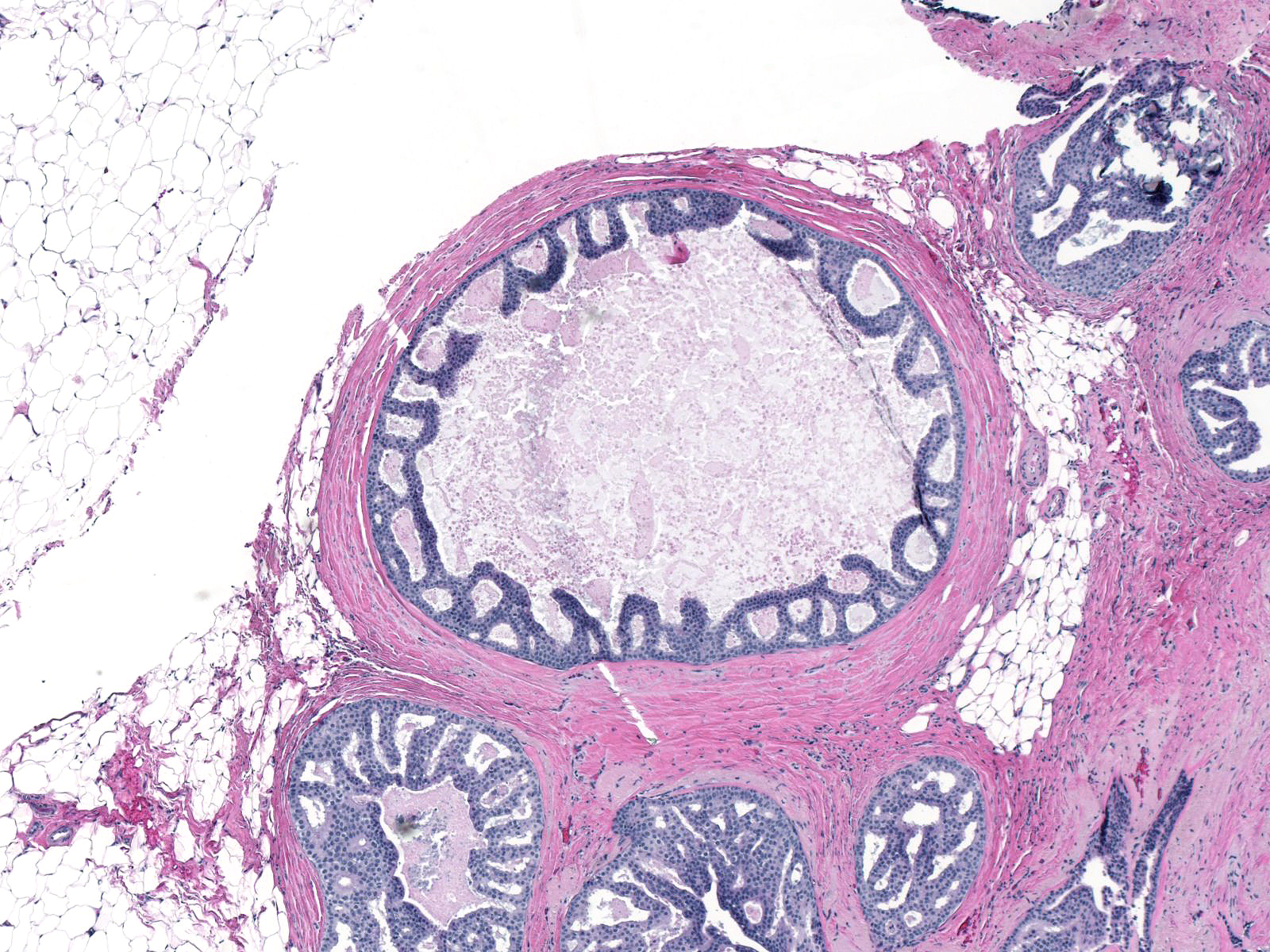
| Besides displacing the indigenous epithelial cells, the malignant ductal cells can grow within the epithelium in a pagetoid pattern. |
 |
|
|
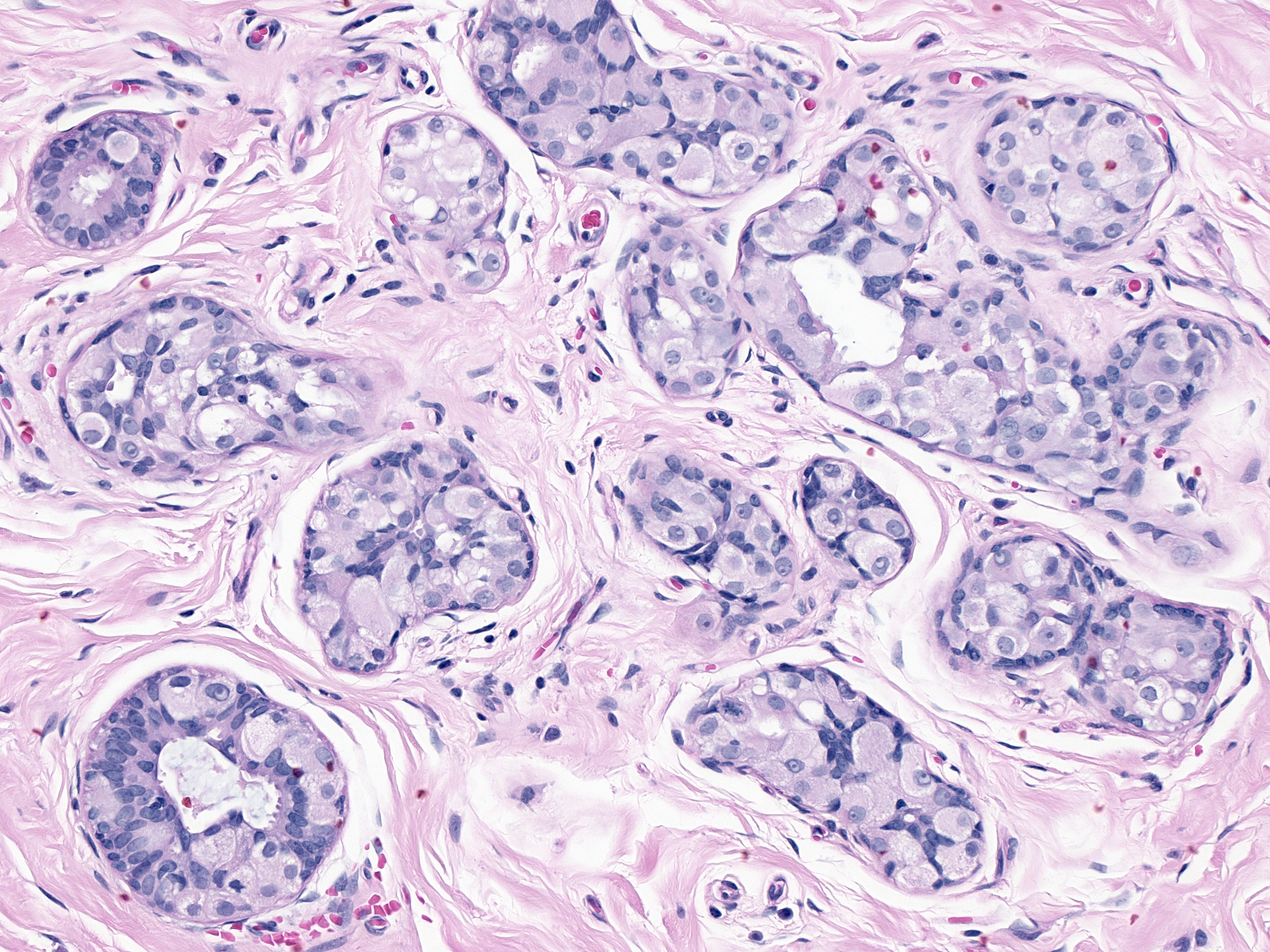
Neoplastic ductal cells appear somewhat dishesive. They usually stick together more than the dishesive cells of lobular neoplastic do, but ductal carcinoma cells do not display the marked cohesion characteristics of hyperplastic ductal cells. This lack of cohesion allows the cells to fall into the lumen of the affected duct and into the spaces formed by the neoplastic cells.
To make the diagnosis of DCIS, one must integrate the histological findings according to a logically coherent analysis. It begins with an assessment of the presence of cytological atypia. The absence of cytological atypia excludes the presence of all forms of ductal neoplasia. If the cytological atypia appears high‑grade, one can make the diagnosis of malignancy without regard to other features of the population. If the cytological atypia appears intermediate‑grade, one need to observe only minimal architectural to make the diagnosis of carcinoma. When one cannot detect architectural atypia, one is forced to use the diagnosis of ADH. Because pathologists typically reserve the diagnosis of ADH for proliferations showing low‑grade atypia, the use of that diagnosis for proliferations showing intermediate grade atypia is unsatisfying. If the cytological atypia appear low-grade, one must observe well established architectural atypia and a robust proliferation to make the diagnosis of DCIS. Flat proliferations merit the diagnosis of flat epithelial atypia; those showing only minimal architectural atypia or occupying only a minute region merit the diagnosis of ADH.
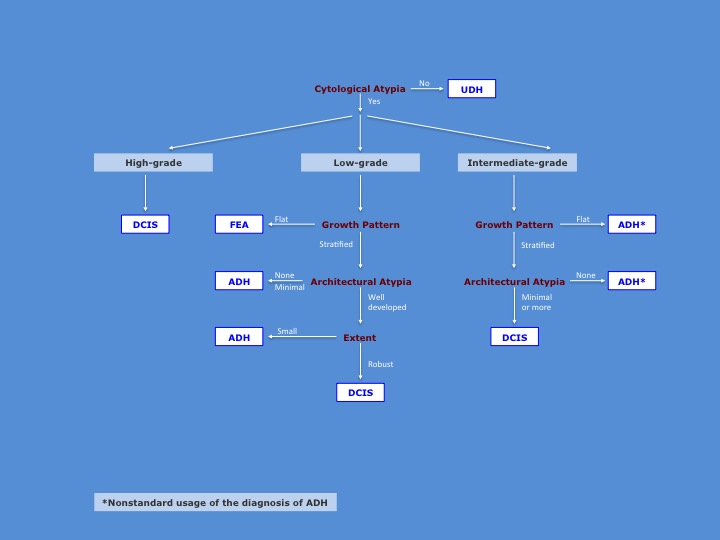 |
| Here is an illustration of the diagnostic algorithm. |
Breast Pathology



































