Fibrocystic changes (FCC) refers to a collection of benign changes featuring either intralobular fibrosis or cyst formation or both.
Contents
Introduction
|
Definition: Fibrocystic changes develop in women during their reproductive years and usually regress after menopause. FCC can present on physical exam as an ill-defined mass, a region of firm tissue, or as one or more cysts. They can also present during radiographic screening as an irregular density or indeterminate calcifications. Clinical Significance: Fibrocystic changes appear as a poorly defined, irregular region of firm, white tissue and/or cysts of varying size. Often the cysts and the firm white tissue mingle. The cysts contain clear, pale yellow, or red-brown fluid. Those containing red-brown fluid appear blue when they are intact and go by the name of blue dome cysts. For additional description, see the FCC portion of the basic gross pathology page. Gross Findings: The diagnosis of fibrocystic changes requires the presence of either intralobular fibrosis or cyst formation. Intralobular fibrosis is characterized by replacement of the specialized intralobular stroma by dense collagen. It is often accompanied by shrinkage of the associated acini and condensation of the surrounding basement membranes. The epithelial cells lining the cysts of FCC often appear flat, but they can be cuboidal or columnar. They do not appear atypical. Microscopic Findings: The differential diagnosis for FCC depends on the component. One could confuse cases in which fibrosis dominates with examples of pseudoangiomatous stromal hyperplasia (affects non-specialized stroma), surgical scar (usually replaces the glandular element), radiation changes (look for other radiation changes), and diabetic mastopathy. (Diabetic mastopathy is a rare lesion featuring glandular atrophy, stromal fibrosis, and dense lymphocytic infiltration.) Differential Diagnosis: Pathologists differ in the histological alterations categorized as fibrocystic changes. Common teaching divides fibrocystic changes into two categories: nonproliferative (fibrosis, cysts, apocrine change, adenosis, and mild usual ductal hyperplasia) and proliferative (moderate or marked usual ductal hyperplasia, sclerosing adenosis, radial scar, and papilloma). Pathologists at MGH do not distinguish the two classes of fibrocystic changes when reporting the diagnosis, nor do they include sclerosing adenosis, radial scars, and papillomas under the rubric of fibrocystic changes. As used at MGH, the diagnosis of fibrocystic changes refers to the presence of either intralobular fibrosis or cyst formation and may also include apocrine change and usual ductal hyperplasia Discussion: Fig_4-2.jpg |
Intralobular Fibrosis
| This lobule demonstrates intralobular fibrosis, shrinkage of acini, and condensation of the basement membranes. | 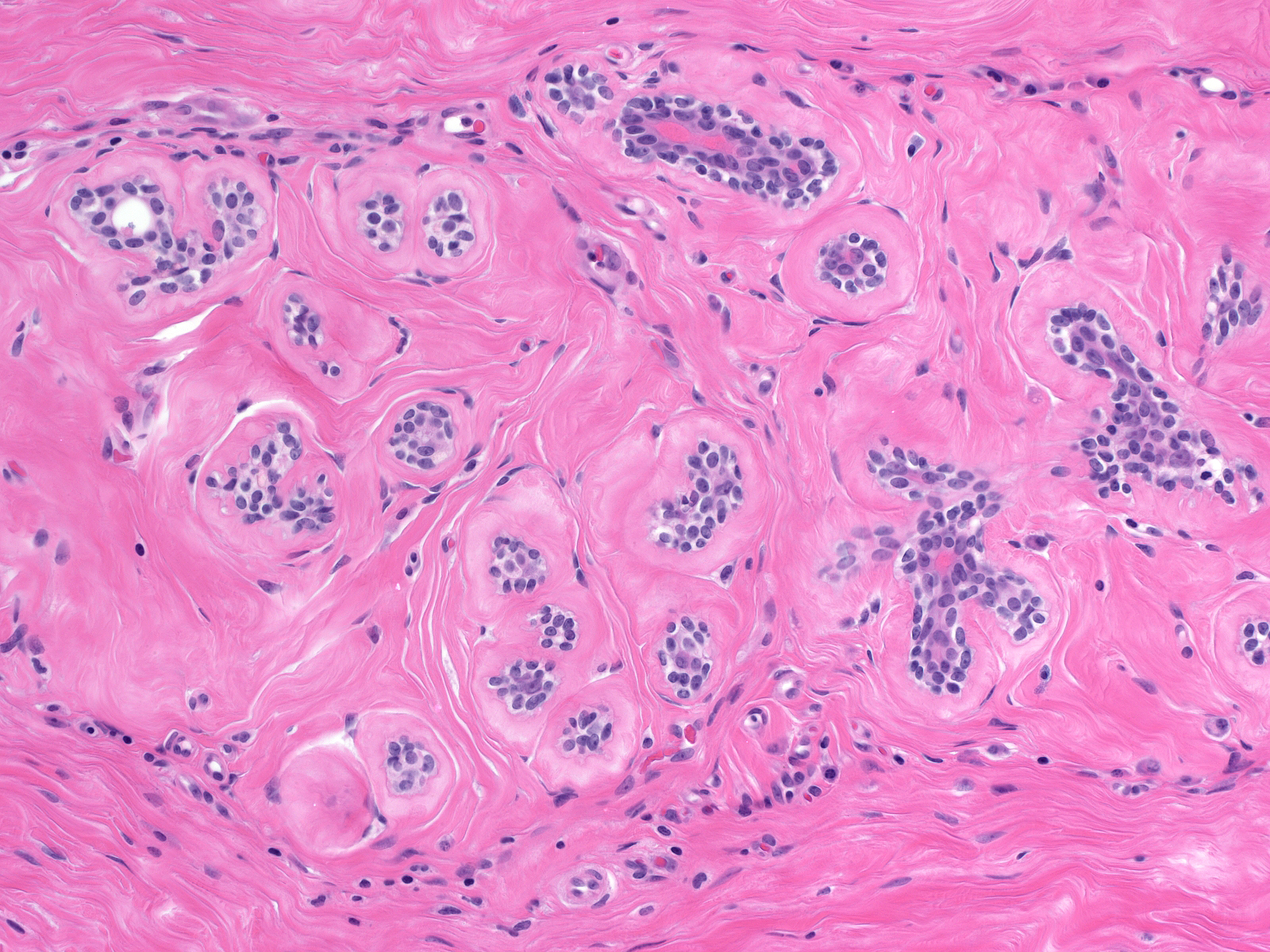 |
Cyst Formation
| Dilatation of acini represents an early stage in the formation of microcysts. Most cysts form in acini as a consequence of an imbalance in secretion and reabsorption. | 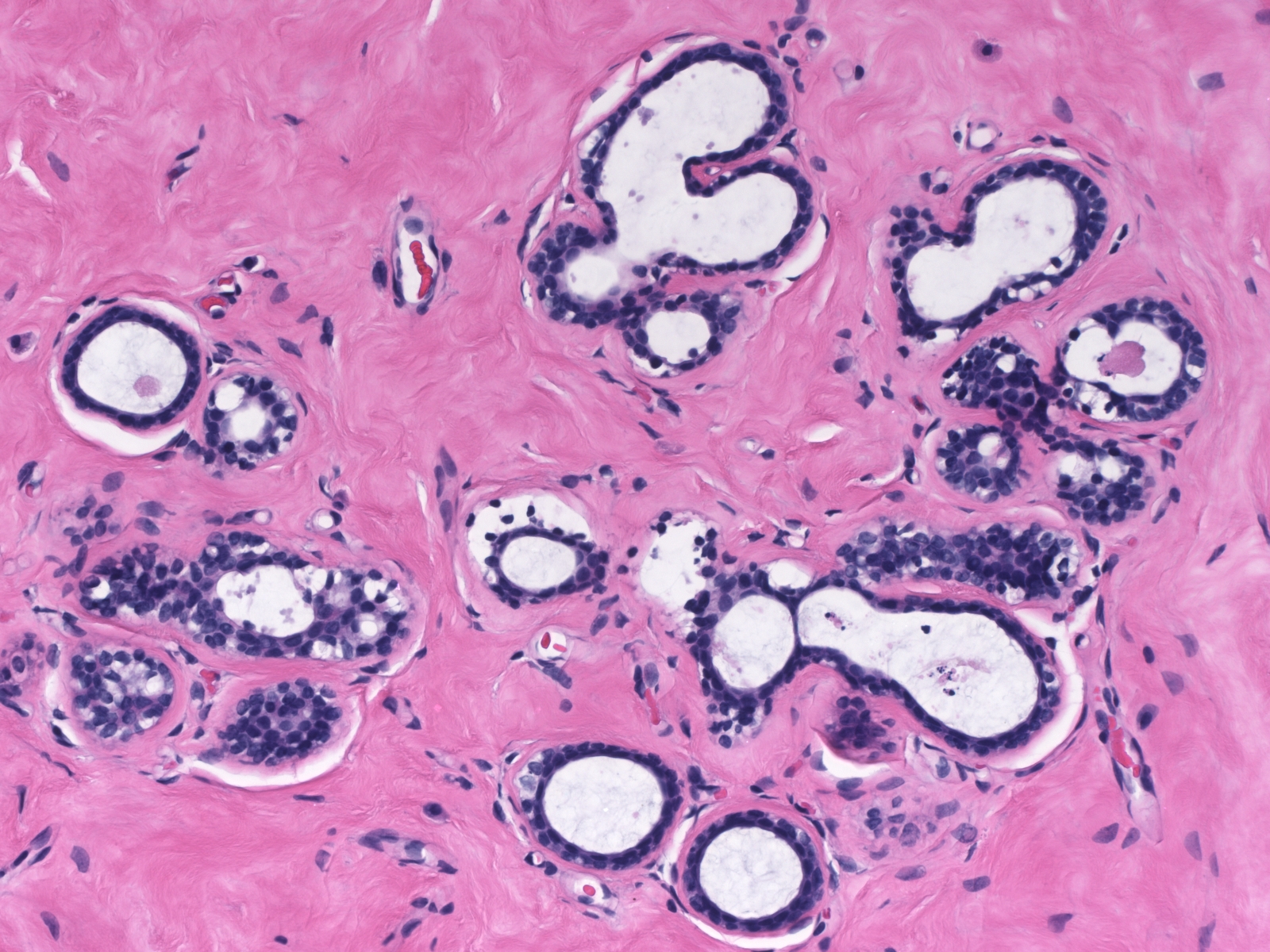 |
| Since the increase in the size of the cysts is not necessarily accompanied by cellular proliferation, the epithelial cells forming the lining often attenuate and appear flat, but they can display cuboidal or columnar shapes. In macrocysts, the epithelial lining often disappears entirely, leaving only circumferentially arrayed, dense collagen. Chronic inflammatory cells sometimes accumulate in the tissue surrounding these cysts. | 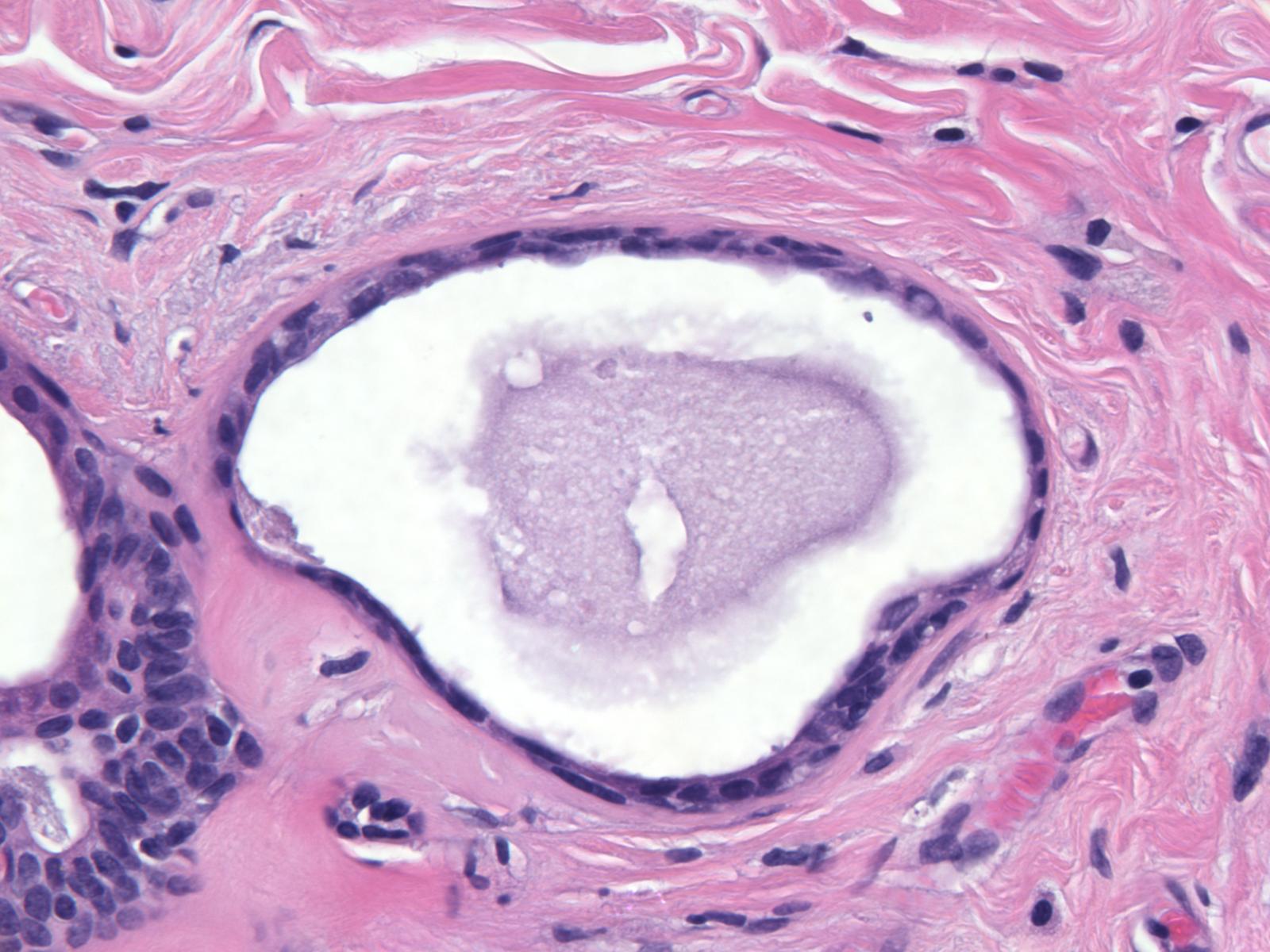 |
Apocrine Metaplasia
| Apocrine metaplasia underlies the formation of many cysts. In this example, the shapes of apocrine cells vary from flat (left) to columnar (right). | 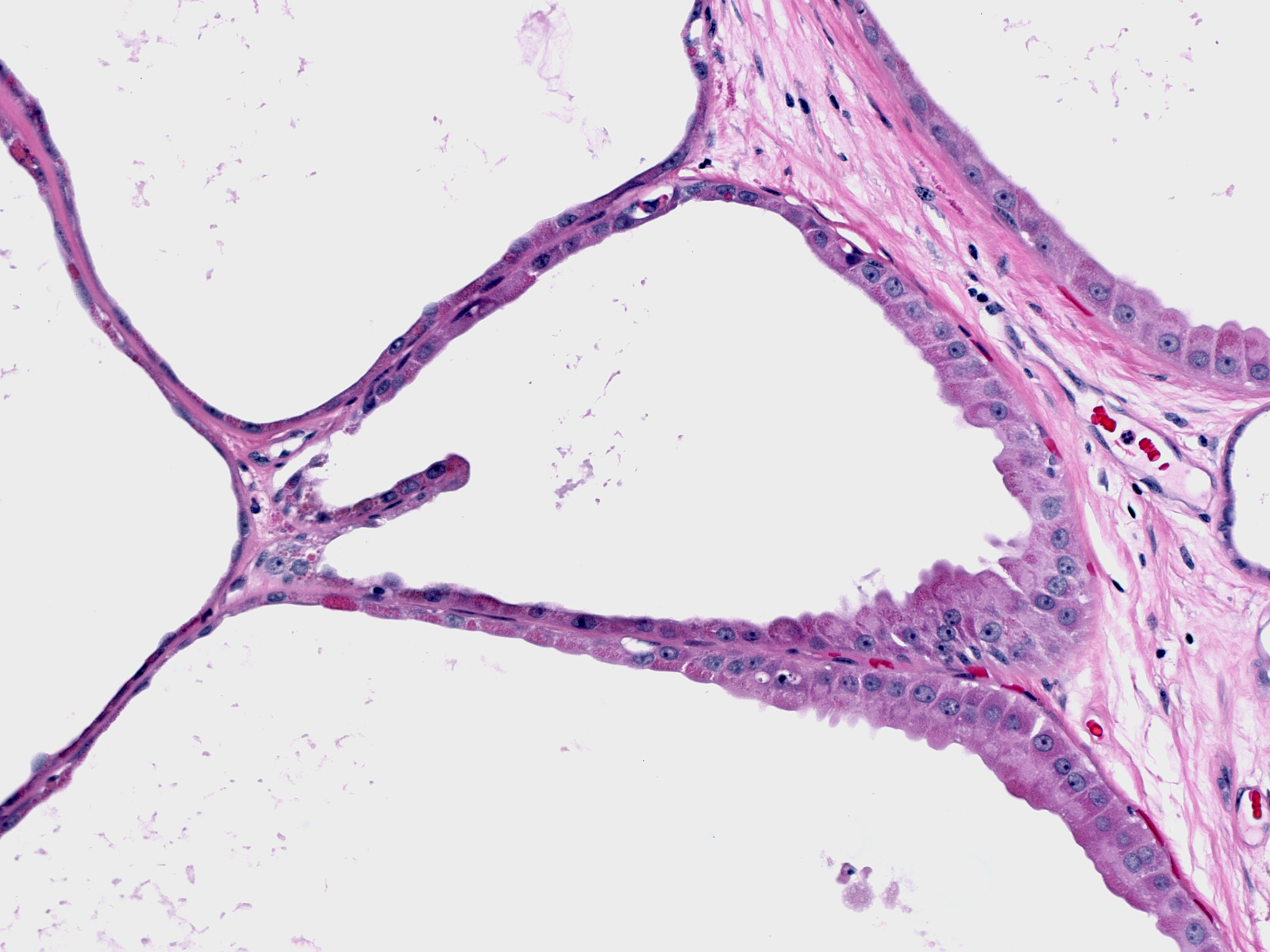 |
Calcifications
| Calcium hydroxyapatite (basophilic) can crystalize within the cysts of FCC, or in the stroma immediately adjacent to the cysts. |  |
| Nearly colorless crystals of calcium oxalate sometimes occupy the lumens of apocrine cysts. In this example, the lining cells appear so flattened it is hard to recognize their apocrine nature. | 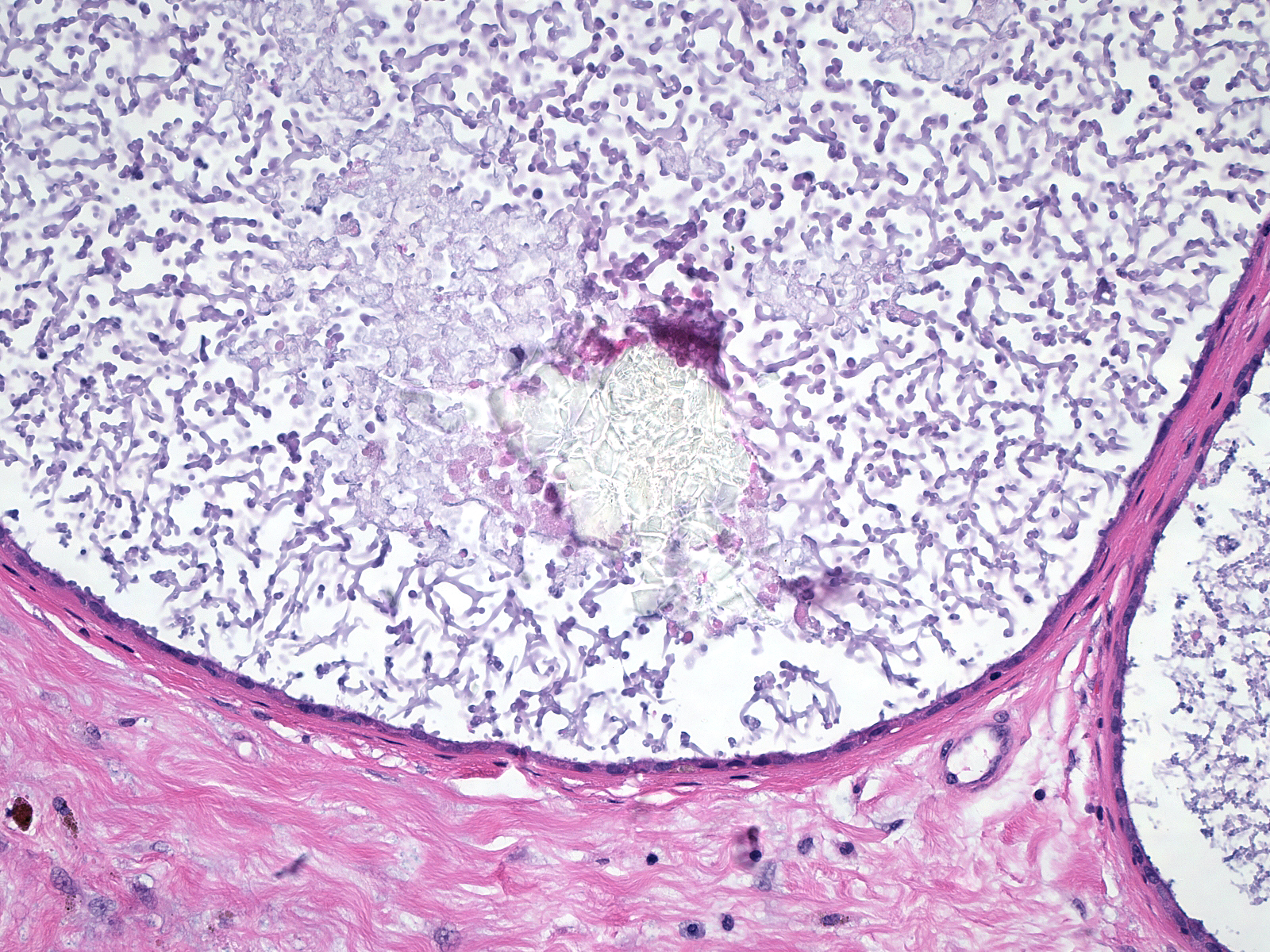 |
Quiz