Difference between revisions of "mgh:apcallfaq"
| Line 6: | Line 6: | ||
<big> '''PLEASE CONTACT Nicholas Caldwell (njcaldwell@mgh.harvard.edu) WITH ANY SUGGESTIONS FOR UPDATES/EDITS''' <big> | <big> '''PLEASE CONTACT Nicholas Caldwell (njcaldwell@mgh.harvard.edu) WITH ANY SUGGESTIONS FOR UPDATES/EDITS''' <big> | ||
| + | *'''REMINDER''': B+ waste (used during lymphoma work-up) needs to be discarded into a secondary container labeled “B+ waste". The B+ waste is located on the bottom shelf in the flammable cabinet in the frozen lab (the same cabinet where the B+ is located). ('''01/14/2024''') | ||
*Added information about Stage Mohs Excision grossing in Prepping > Dermatopathology section. Per dermatopathology staff consensus: "Having the slides ready the next day morning is very important so we can obtain deeper sections and rush immunostains if needed. Likely a very rare scenario, but if a slow Mohs or staged excision arrives after 6pm during a weekday, having it grossed that evening and be processed overnight will be best for the patient. For case arrived late on Friday, it does not need to be processed for Saturday. It needs to be grossed and be processed overnight on Sunday so we can have the slides first thing Monday morning." ('''12/05/2024'''). | *Added information about Stage Mohs Excision grossing in Prepping > Dermatopathology section. Per dermatopathology staff consensus: "Having the slides ready the next day morning is very important so we can obtain deeper sections and rush immunostains if needed. Likely a very rare scenario, but if a slow Mohs or staged excision arrives after 6pm during a weekday, having it grossed that evening and be processed overnight will be best for the patient. For case arrived late on Friday, it does not need to be processed for Saturday. It needs to be grossed and be processed overnight on Sunday so we can have the slides first thing Monday morning." ('''12/05/2024'''). | ||
*Added information about MEE specimen banking after hours ('''12/04/2024'''). | *Added information about MEE specimen banking after hours ('''12/04/2024'''). | ||
Revision as of 13:47, January 14, 2025
Contents
- 1 Recent Updates
- 2 Pager Operation (MGH Anatomic Pathology)
- 3 GME Call Rooms
- 4 Sharps, Needlestick, Blood Exposure Workflow
- 5 Overview of MGH Call Responsibilities
- 6 Weekday Junior Call Responsibilities
- 7 Weekday Senior Call Responsibilities
- 8 Weekend Junior Call Responsibilities
- 9 Weekend Senior Call Responsibilities
- 10 Call Skills
- 11 Prepping
- 12 Uncommon / Unique Frozen Section Scenarios
- 13 General Hematopathology Resources
- 14 Courier Instructions
- 15 Cytology After Hours: ROSE, Accessioning, WRN1 refrigerators, and Processing
- 16 Autopsy After Hours: Request for Cases, NEOB inquiries, etc
- 17 Banking Tissue for Research
Recent Updates
PLEASE CONTACT Nicholas Caldwell (njcaldwell@mgh.harvard.edu) WITH ANY SUGGESTIONS FOR UPDATES/EDITS
- REMINDER: B+ waste (used during lymphoma work-up) needs to be discarded into a secondary container labeled “B+ waste". The B+ waste is located on the bottom shelf in the flammable cabinet in the frozen lab (the same cabinet where the B+ is located). (01/14/2024)
- Added information about Stage Mohs Excision grossing in Prepping > Dermatopathology section. Per dermatopathology staff consensus: "Having the slides ready the next day morning is very important so we can obtain deeper sections and rush immunostains if needed. Likely a very rare scenario, but if a slow Mohs or staged excision arrives after 6pm during a weekday, having it grossed that evening and be processed overnight will be best for the patient. For case arrived late on Friday, it does not need to be processed for Saturday. It needs to be grossed and be processed overnight on Sunday so we can have the slides first thing Monday morning." (12/05/2024).
- Added information about MEE specimen banking after hours (12/04/2024).
- Added information about After-hours lymphoma workup specimens. Per hematopathology staff consensus: all rush and top priority cases should be submitted same day. Other specimens designated routine can wait until the next morning unless the senior on-call feels it should be loaded same-day. (11/15/2024).
- Added PDF detailing new specimen ordering screens (as a result of Beaker implementation) to go live 11/03/2024; see Call Skills > Covering the #23305 pager. (10/31/2024).
- Added language about 1:00pm Sunday junior resident prepping cut-off (Weekend Junior Call Responsibilities). Added reminder to check placenta/POC requisitions during weekend call prior to move to fridge. (08/29/2024).
- Added information about changes to transbronchial biopsy processing (all tissue is now submitted in 1 container); see Prepping > Uncommon Biopsies for more info (08/21/2024).
- The layout of this page has been rearranged to allow for easier navigation & expansion. No information has been removed, just rearranged. Let the AP chief resident know if you want to see any additions to this page (08/20/2024).
- Added back-up copy of text within this document to pat_pat1$ ( \\Cifs2\pat_pat1$\Resident AP Call Info Back-Up ) (08/08/2024).
- Updated Senior Weekend Call Responsibilities, to include linked MediaLab SOPs, reflecting SOP language, and new weekend RUSH scanning availability (08/08/2024).
- Pager Operation and GME call rooms sections expanded (07/24/2024; 07/25/2024).
- After Hours Cytology Updates: Re-wrote cytology after-hours policy to highlight relevant MediaLab SOPs, updated policies following senior resident ROSE credentialing, and T2000 processing. Changes reviewed & edited by Dr. Chebib. Please contact AP chief resident if additions or clarifications needed (07/24/2024).
- Flow Cytometry Label Reminder: Please double check that the correct label is placed on all flow send out tubes. It MUST be the container label that indicates the part type (A, B, C, ...) of the specimen; it CANNOT be the requisition label. Placed in "Lymphoma Work-up" section (07/13/2024).
Pager Operation (MGH Anatomic Pathology)
- Paging across MGH/BWH is done using the Phone & Paging Directory (LINK HERE)
- Forwarding a pager can be done online or with the hospital operator
- Online: Once you have “Change Paging Status” access (see more info below), go to the Phone & Paging Directory → search 23305 → within “Change Paging Status”, select "7 - Calls Being Taken by Page ID" in the dropdown menu → click "File Change" → enter your personal pager number → "File"
- Hospital Operator (in-house: dial “0”; out-of-house: call 617-726-2000)
- Ask “Can I forward a pager, please”; then respond with pager you want to forward (for AP residents, “23305”); then respond with pager you want to forward to (your own personal pager number).
- Scheduling Pager Forwarding for On-Call Shifts
- Please DO NOT use the pager forwarding scheduler with the #23305 pager (MGH Anatomic Pathology First Call). This feature is intentionally not used to ensure that MGH frozen section & on-call service hand-offs are deliberate and in-person.
- How to get “Change Paging Status” access for shared pagers.
- Uses: Most frequently used when “taking over” #23305 (MGH Anatomic Pathology, 1st Call) when taking Junior call. Will be used for other pagers on other services, which you can get access to as needed for your rotations.
- How to: Email isppdedits@partners.org to request access.
- Example Email: “My name is _____ and I’m a pathology resident at MGH. Could I please get access to the #23305 pager?”
- How to forward pages to your email:
- From home page → Click “Go to my profile” (top right)
- To the right of “Page ID: #####” (within square on top right of page), there is a square with an overlaid pencil; click on that square.
- You’ll be taken to a new page; the bottom item of a small list is a line stating “Copy pages via email?”. Check off this box, then select “File”.
- Seeing pages you’ve received:
- 1) Can look through email if you opted into getting pages forwarded to your email
- 2) Can click blue “Retrieve Msgs” button near the right / top of your My Profile page.
- Seeing pages you've sent:
- Click the “View” button on the left side of the My Profile page next to “Pages Sent By This User”
- Batteries:
- AA batteries are provided for our pagers by the department. These are stored in the office supply cabinet at MGH in the WRN2 resident room. If no batteries are here, please contact the AP chief resident.
- Empty AA batteries are recycled. These can be returned to the “dead battery cup” within the office supply cabinet in the WRN2 resident room.
- Etiquette:
- Please carry your pager throughout the workday and answer promptly.
- If you leave your pager at your desk (for the night, for vacation), silence / turn-off your pager to avoid it alerting when you are not able to answer. Constant unanswered alerts are disruptive for your fellow residents.
- In the “Change Paging Status”, there is an option to add a Paging Note to your profile. This note will appear whenever someone is about to page you. Up to you what you write, but an option could be the following:
- “Please page #23305 (MGH Anatomic Pathology, on-call resident) outside of 800a-600p for surgical pathology questions.”
GME Call Rooms
The Graduate Medical Education (GME) provides call rooms for programs that do not have dedicated call rooms (like pathology). There is no official GME policy about the use of these rooms or any points of etiquette. Pathology trainee experience with these call rooms is extremely limited; the availability of these rooms is likely too unreliable to be given serious consideration for using. It is unclear how often or when the rooms are cleaned and bedding is turned over. If you do end up using one of these rooms, please let the AP chief resident know about your experience. (07/25: MGH AP chief resident has requested more information from GME)
- Men: Located in Wang 1 (Room 125): Off-shoot room of the general GME trainee lounge; 1 bunk available.
- Women: Located in Bigelow 9 (Room 952): Combined lactation room & call rooms; 2 bunks available.
Sharps, Needlestick, Blood Exposure Workflow
See link to protocol for MGH staff (LINK HERE; 06/24/2024) that details flow diagram for "Employee sustains sharp injury or mucous membrane exposure".
- First wash the area thoroughly
- Then, promptly contact occupational heath
- Business Hours (M-F, 7a-5p; non-holidays): Call 617-726-2218
- Afterhours: Page #21272
- Refer to flow diagram for further information
Overview of MGH Call Responsibilities
Senior and Junior Residents: Exchange contact information with each other prior to taking call.
Please DO NOT use the pager forwarding scheduler with the #23305 pager (MGH Anatomic Pathology First Call). DO NOT set an "end-time" for your #23305 pager coverage. The pager forwarding scheduler service is intentionally not used to ensure that MGH frozen section & on-call service hand-offs are deliberate and in-person.
Hours
- Weekday Junior Resident
- Covers #23305 pager (Pathology MGH 1st Call) and performs on-call duties from 6:00pm to 8:00am.
- Required to be in hospital from 6:00pm to 8:00pm; after this time, in hospital as needed to handle call responsibilities.
- Will rarely be asked to help cover the frozen lab from 5:00pm to 6:00pm in situations when the on-call senior resident is the same as that day's frozen section senior resident.
- Weekday Senior Resident
- Covers frozen section interpretation and performs on-call duties from 5:00pm to 8:00am; supervision of Weekday Junior Resident from 6:00pm to 8:00am.
- Required to be in hospital from 5:00pm to 8:00pm; after this time, in hospital as needed to handle call responsibilities.
- Weekend Junior Resident
- Covers #23305 pager (Pathology MGH 1st Call) and performs on-call duties from 8:00am Saturday to 8:00am Monday.
- Required to be in hospital from 8:00am Saturday to 2:00pm Saturday; after this time, in hospital as needed to handle call responsibilities.
- Sunday prepping: As of 08/30/24, we are trialing a discrete "cut-off" time for prepping specimens on Sunday. All specimens that arrive in the Blake 354 OR refrigerator prior to 1:00pm need to be prepped by the on-call junior resident. Use clinical judgement when deciding the need to prep specimens after this time.
- Weekend Senior Resident
- Covers frozen section interpretation and performs on-call duties from 5:00p Friday to 8:00am Monday; supervision of Weekday Junior Resident from 6:00pm Friday to 8:00am Saturday; supervision of Weekend Junior Resident from 8:00am Saturday to 8:00am Monday.
- Required to be in hospital from 8:00am Saturday to 2:00pm Saturday; after this time, in hospital as needed to handle call responsibilities.
- AP Chief Resident
- Will rarely be asked to help cover the frozen lab from 5:00pm to 6:00pm in situations when the on-call senior + junior resident is the same as that day's frozen section senior + junior resident.
Swapping Call Days
- In non-emergent situations, the resident desiring a call swap organizes and confirms swap with another resident. Junior Call Residents should reach out to other Junior Call Residents; Senior Call Residents should reach out to other Senior Call Residents.
- Email MGH AP Chief Resident requesting change; they will update the call schedule. The change in schedule is not immediately apparent on LearnPath - it takes a short time for updates to be pushed to LearnPath.
Senior versus Junior Call Eligibility
- MGH Senior Call is taken by residents starting the spring/summer before their final year (end of PGY2 for AP-only; end of PGY3 for AP/CP) after successful completion of 1) the frozen section conference and 2) frozen section credentialing.
- MGH Junior Call is taken by resident who have not completed Senior Call conference and credentialing.
- Weekday Junior Call eligibility begins after completion of 1-week of the Frozen Section Junior Resident rotation.
- Weekend Junior Call eligibility begins during PGY2 year.
Weekday Junior Call Responsibilities
Starting your call night
- Report promptly to the frozen section lab at 6:00pm.
- Transfer the #23305 pager to your personal pager (often done ~5:50-5:55pm before coming up to the frozen lab). See Paging Operation section for more details.
- Hand-off with the daytime Frozen Section resident:
- Ask the frozen section resident about any ongoing cases. If the frozen section resident is still busy at 6pm, make every effort to relieve them close 6:00pm. This may involve helping them finish the frozen they are currently working on, accessioning cases that may have recently come in, put chucks into cassettes, etc.
- The frozen section resident SHOULD NOT leave without making sure that all the specimens from the day have gross descriptions!
- If frozen sections on a chuck are still in the cryostat and have not been taken down, it is the FROZEN SECTION RESIDENT’S RESPONSIBILITY to make sure that they are clearly labeled with the patient’s name and frozen section number. If they are not clearly labeled, the frozen section resident is responsible for taking these frozens down, labeling them, and putting them with the correct case even if it is after 6pm.
Carry the #23305 Pager
- Carried from 6:00pm to 8:00am.
- Do not set an "end-time" when transferring your pager!
- Refer to "Covering the #23305 Pager" section for more granular tips.
- If any issues arise that you are unsure as to how to handle, page your senior resident.
Assist in the Frozen Section Lab with Intraoperative Care
- During weeks on Frozen Section, junior residents will spend most of their time grossing specimens, writing gross descriptions, and placing tissue on chucks. During weeknight call, junior residents need to be prepared to do all of that, plus use the cryostat and stains to prepare frozen section slides!
- 6:00pm: Typically, there will be 1-2 cases wrapping up from the late afternoon. Be in the lab promptly at 6:00pm to transfer care and help with these cases.
- 6:00pm to 8:00pm: The surgeons expect us to be available in-house until 8pm so that there is essentially no delay between the time they call and the time we are available to do a frozen. This time is typically spent in the frozen lab processing specimens, answering pages, and, frequently, prepping breast specimens.
- After 8:00pm: If any frozens come in, the surgeon will page #23305 and should be understanding when you tell them that we are not in-house and to anticipate a 30-40 minute delay until the frozen can be performed.
- In general, it is better to page the senior resident when a specimen comes in before cutting into it – they are less experienced at interpreting frozen sections than the attendings who cover the lab during the daytime, and often prefer to evaluate the specimens for themselves and help with selection of sections for frozen section processing.
Prep Breast Specimens
- Background: Throughout the afternoon, the PAs & residents are grossing breast specimens "fresh" to be processed early the next morning. Occasionally, breast specimens will arrive later in the afternoon (generally after 5:00pm) such that there is not enough time left in the day for the specimen to be grossed in. In those events, the left-over breast specimens from the day need to be prepped (not grossed) by the on-call junior resident.
- You will be notified via email about the specimens needing attention by 6:00pm by the PAs.
- A guide to prepping these specimens can be found on the AP Call Info Page (Prepping > After-Hours Breast Specimen Prepping) to include an SOP.
- If you have any questions or are unsure, contact the on-call senior resident!
Checklist of Tasks prior to Leaving (8:00pm)
- REMOVE the blade in the Cryostat.
- Turn off the Cryostat light and shut the cryostat lid/door. Cover alcohol jars.
- Cover all staining materials.
- Clean up blood and tissue from Frozen section benches.
- Make sure all used frozen section chucks with tissue in them have been removed from the Cryostat and put into a cassette (labeled with FX sticker) and are placed in a container with formalin OR saved frozen tissue should be placed in a labeled bag and put in the -80C fridge in the histology lab to go to Warren 5.
- Do not leave frozen tissue in the cryostat overnight. The cryostat has a thaw cycle and the specimen will be damaged.
- Empty all cubbyholes above the staining bench and place specimens in the “open” cubbyholes above the cubbyholes where specimens to be grossed by residents are kept.
- After accessioning new cases, write them in the logbook (place accession sticker on front page with # of frozens done) and place photocopy the requisition sheet in the folder with the frozen slides.
- Turn microscope and room lights off.
Make sure the next day's Frozen Section Junior resident has picked up the #23305 pager
Weekday Senior Call Responsibilities
- Covers frozen section interpretation and performs on-call duties from 5:00pm to 8:00am; supervision of Weekday Junior Resident from 6:00pm to 8:00am.
- Required to be in hospital from 5:00pm to 8:00pm; after this time, in hospital as needed to handle call responsibilities.
- Supervises weekday junior resident with their responsibilities.
Weekend Junior Call Responsibilities
Overview
- Covers #23305 pager (Pathology MGH 1st Call) and performs on-call duties from 8:00am Saturday to 8:00am Monday.
- Required to be in hospital from 8:00am Saturday to 2:00pm Saturday; after this time, in hospital as needed to handle call responsibilities.
- Responsibilities include:
- Covering the 23305 pager
- Triaging/prepping specimens
- Processing RUSH/top priority specimens
- Assisting in the frozen section lab for intraoperative consults
- Loading the processor on Saturday and/or Sunday
- Prior to taking MGH weekend call for the first time, please coordinate with the current AP chief resident for formal onboarding. Before attending the onboarding session, review the following documentation.
- See sections below for more in-depth formation.
Covering #23305 Pager
- Refer to "Covering #23305 Pager" section above for more information.
Triaging & Prepping Specimens
- General Goal: Weekend triaging of specimens by the resident allows for 1) expedited processing of RUSH / Top Priority cases and 2) maintenance of specimen integrity (placing surgical pathology specimens in formalin after prepping, moving placentas to refrigerator, sending specimens to core lab/micro originally intended for those labs). The senior resident is available and should be called with any questions. Please keep these principles in mind when approaching the weekend.
- The specimens the junior resident is responsible for triaging arrives in the Blake 354 in generally four different ways.
- Through Blake 354 drop-off window (specimens from clinics and inpatient units; generally in formalin, except for placentas)
- Through OR refrigerator (specimens from ongoing surgeries; typically fresh)
- Through OR door (in need of frozen section evaluation)
- Through Core Lab (specimen intended for surgical pathology processes delivered to core lab; if you're looking for a specimen for RUSH processing and it is not in the gross lab, check in with the core lab)
- Deciding if the specimen is for Surgical Pathology (& formalin) or Core Lab/Microbiology (& no formalin)
- Most specimens arriving to the Blake 354 Gross Lab are intended for surgical pathology processing (ie formalin fixation, grossing, loading onto a processor). Frequently, however, specimens intended to be processed in the core lab or microbiology lab arrive in the surgical pathology lab. Specimens in the surgical pathology lab intended for core lab / microbiology lab should be carried there in person as soon as possible. Both the core lab and microbiology labs are located on Gray 5.
- How do you know if a specimen is meant for the core lab or microbiology lab?
- Specimens intended for core lab or microbiology testing are labeled with a "different" sticker from what is typically seen on surgical pathology specimens.
- See photo example of Clinical Pathology / Sunquest sticker.
- Review the patient's laboratory orders in EPIC. Does the patient have a surgical pathology order? Or are there only clinical pathology orders present?
- "Sneaky" examples of clinical pathology specimens include fresh tissue for microbiology culture, gallstones/urinary stones for stone analysis, liver cores for hemochromatosis testing, etc.
- If you are unsure if a specimen belongs in the core lab or surgical pathology lab, you can reach out to your senior resident or the core lab to clarify.
- Specimens intended for core lab or microbiology testing are labeled with a "different" sticker from what is typically seen on surgical pathology specimens.
- Blake 354 Drop-Off Window: Generally, specimens from clinics and inpatient units; generally, arrive in formalin (except for placentas)
- Move placentas and POCs to Blake 354 OR refrigerator 'bottom shelf. These do not come to the lab in formalin, and we do not put formalin in them prior to transferring to the refrigerator.
- Review placenta requisitions prior to moving to fridge! It is important to review priority status (RUSH or not) and need for ancillary studies.
- There is no need to gross placentas (NICU or otherwise) unless contacted and explicitly asked by Drucilla.
- POCs generally should arrive in the OR refrigerator, however, may arrive at the drop-off window (see POC section below for more information).
- Check specimen requisitions to identify any RUSH or Top Priority cases.
- Generally, you are made aware of RUSH specimens prior to their drop-off at the window. Rarely, RUSH specimens will be found in the basket without a prior notification.
- Process any RUSH or Top Priority case that arrives in this basket (see Processing RUSH & Top Priority Specimens section below for more considerations).
- Surgical pathology techs will be working most Saturdays and will often triage specimens that arrive in this basket. However, still consider any specimen you see in this basket as non-triaged and review.
- Move placentas and POCs to Blake 354 OR refrigerator 'bottom shelf. These do not come to the lab in formalin, and we do not put formalin in them prior to transferring to the refrigerator.
- OR Refrigerator: Generally, specimens from the operating room; generally arrive fresh
- This is the source of the majority of the triaging/prepping weekend work. All new specimens in this refrigerator need to be triaged/prepped.
- When you arrive in the lab on Saturday morning, take a moment to review & organize the contents of the refrigerator. This is important so that you are able to recognize when a new specimen arrives. Although items may shift around, the general organization of the refrigerator is: Bottom - Placentas, Large Specimens; Middle Two Shelves - new specimens + specimen "log-in" book +/- cytology bin +/- research/patient samples; Top Shelf - research/patient samples.
- After hours and on the weekends, the specimen "log-in" book is moved from the OR window to the refrigerators. You can use this book to see what new specimens have arrived after hours.
- See separate section for prepping specifics per subspeciality. Ask your senior residents if you have any questions.
- Don't forget to put formalin stickers on your specimens!
- Leave prepped specimens on the table outside of Jenn/Sarah's office / underneath the grossing lists.
- OR Door: Frozen Sections - refer to appropriate sections below.
- Core Lab: Residents frequently communicate with the core lab over the weekend to identify the location of RUSH specimens. If you're looking for a specimen for RUSH processing and it is not in the gross lab, check in with the core lab. Covered more in detail in RUSH / Top Priority processing below.
See separate section below for prepping specifics
Processing RUSH & Top Priority Specimens
- Similar to weeknights, RUSH or Top Priority should be accessioned, grossed, and processed. There are some weekend considerations. See specific sections for overview.
- Reminders:
- Neuropath specimens (except temporal lobe excisions or other large excisions) and other small specimens that have a frozen section performed on the weekend are typically processed for a Monday morning read.
Assisting in the Frozen Section Lab
- During weeks on Frozen Section, junior residents will spend most of their time grossing specimens, writing gross descriptions, and placing tissue on chucks. During weeknight/weekend call, junior residents need to be prepared to do all of that, plus use the cryostat and stains to prepare frozen section slides!
- Refer to the Call Skills > Frozen Section Cutting section for tips.
Loading the processor on Saturday and/or Sunday
- In addition to loading the processor as needed for RUSH specimens, the on-call junior resident is responsible for loading the cassettes for any cases grossed in over the weekend. Weekend staffing of PAs and techs is variable, but usually there is some sort of staff present that is producing cassettes from grossing.
- Loading the processor is typically done on both Saturday and Sunday. Before your weekend call, it is helpful to talk to the lab managers (Jenn Patel and Sarah Ferguson) to confirm what staff will be working over the weekend. When you arrive to the lab on Saturday, check in with whatever staff is present to see how long they'll be working and when you'll be able to load their cassettes.
- The techs in small gross will most likely load their own cassettes, however, do confirm this with them before assuming they will.
- If the resident grosses a few small RUSH cases and Top Priority cases Saturday morning, you can often ask to get these cassettes "on" with the tech cases to save on processor space.
- Refer to the Call Skills > Loading the Processor section for tips.
Note about Holiday Weekends
- As on non-holiday weekends, timing is from 8 am the first day (typically Saturday) until 8am the next business day.
- On holiday weekends, usually the processors run once during the weekend and then the evening before the next business day. Check with the histology lab on Friday when the processors will run over the weekend before making your plans to load. In general, the processors should be loaded once before the first run, and then the day before the next business day (so if the holiday day is Monday, on Saturday and Monday, with Sunday being optional). Check the decal rack before loading.
Weekend Senior Call Responsibilities
Before taking call, please exchange contact information with Junior Residents.
(1) Weekend/Holiday RUSH cases
- SOP 111736.3265 Policy on Weekend/Holiday RUSH Cases (LINK HERE)
- Friday Triaging General Steps:
- All RUSH cases accessioned after 10 am on Friday will be accessioned to the weekend senior on-call resident and the weekend on-call pathologist. The on-call resident will be notified of the accessioning by the gross lab via email.
- The triaging resident will contact the appropriate clinical staff to determine 1) when a diagnosis is required, 2) whom a diagnosis should be verbally communicated too, and 3) their preferred method of contact
- This information should be communicated to the pathology lab via "replying all".
- If the rush is determined to be for a Monday read, the triaging resident must also include the following week's resident and attending on the email reply and update the accessioning tab in CoPath to reflect the Monday staff.
- Friday Triaging Tips:
- Staying organized is important! Use of Outlook mailboxes, excel sheets, or pen & paper could be beneficial. For each case you're emailed, take note of the accession number and assigned service. Also note (through checkmarks, cross-offs, etc.) when you've 1) sent the initial email out to the clinicians, 2) sent the confirmation email to the gross lab (Saturday versus Monday reads), and 3) updated accessioning within CoPath.
- Email Template to Clinician:
- Hi Dr. ____. Regarding patient ____ (MRN _____) - we received a (specimen type) designated for RUSH processing. Is this case appropriate for a Monday morning pathology interpretation?
- Email Template to Gross Lab (Saturday read) (REPLY ALL):
- Hi all. Regarding S2#-##### - this case is for a SATURDAY read, confirmed with Dr. (Clinician). I've cc'd the on-call (subspecialty service) attending and ensured the CoPath accessioning is correct. Please communicate the preliminary diagnosis to Dr. (Clinician) via (email, page, phone).
- Email Template to Gross Lab (Monday read) (REPLY ALL):
- Hi all. Regarding S2#-##### - this case is for a MONDAY read, confirmed with Dr. (Clinician). I've cc'd next week's (subspecialty service) attending/resident and ensured the CoPath accessioning is updated. Please communicate the preliminary diagnosis to Dr. (Clinician) via (email, page, phone).
- Heart biopsy RUSH cases are always for Saturday reads, even if accessioning accidentally sends it as a TRIAGE case (i.e., you don't actually need to triage it for Monday). The Friday cardiology clinician ordering the biopsies is often different from the weekend cardiology clinician, who may actually want their results sooner.
- Saturday RUSH responsibilities / Slide Scanning
- Slide Scanning - Starting 06/2024, all RUSH slides completed Friday evening and throughout Saturday will be scanned into the Integrated Pathology Viewer and are available for digital/remote review. This process is still being iterated; an initial SOP was created and will be finalized prior to linking here. DRAFT SOP: (LINK HERE)
- Workflow:
- Completed RUSH Slides are placed in the 'Weekend RUSH' cubby in the histology lab (far right bottom cubby in histology lab)
- Intermittently, a scanning tech will retrieve these slides and bring them to the slide scanners in WRN0 (basement) for digitization.
- Following scanning, 1) the slides will be delivered to the assigned staff pathologist's mailbox and 2) an email will be sent to the assigned staff pathologist + resident with a hyperlink to the corresponding IPV page.
- Notes:
- The scanning tech (currently Rachel Simone Cazeau) is currently scheduled to work 10:00am to 6:30pm on Saturday. If you have any RUSH slides that were not originally delivered to the Weekend RUSH cubby, coordinate with her to get the 'missed' RUSH cases scanned. She will accept slides up to a certain point in the evening (?6:00p) so she has time to complete the scan before leaving.
- Full-time IT support is not available on the weekends, so if a case is unable to be scanned, be prepared to revert to showing cases over Teams.
- If you have any feedback, please contact the AP Chief Resident.
- Workflow:
- Senior Resident Previewing Expectations for RUSH cases
- ### Nothing concrete; with new changes to workflow (mainly scanning), the exact role of the senior resident is not well understood. Communicate with your attendings if you are in doubt. I hope to clarify this (NCK) ###
- Slide Scanning - Starting 06/2024, all RUSH slides completed Friday evening and throughout Saturday will be scanned into the Integrated Pathology Viewer and are available for digital/remote review. This process is still being iterated; an initial SOP was created and will be finalized prior to linking here. DRAFT SOP: (LINK HERE)
- New RUSH Cases Accessioned Saturday & Sunday
- As new RUSH are delivered to the Blake 354 gross lab, triage these (with the junior resident) to determine when a read is needed. If a read is needed outside of usual weekend hours (ie late Saturday night, all of Sunday), 1) approve the RUSH request with the on-call attending and 2) coordinate with histology to ensure that someone is in the lab to process the specimen. Since there is no slide scanning after 6:30p on Saturday, the slides will need to be reviewed in person or over Teams.
(2) Supervise Frozen Lab and Interpret Frozen Slides
- Collated helpful senior call resources, by organ system, are found in the following Onedrive folder: LINK HERE. These resources are resident-made helpful tips for specimen interpretation & processing
- Reminders:
- The senior on call should ALWAYS page the neuropathology attending when you are alerted to a potential neuro frozen section after hours and over the weekend during call. Do not only send an email; the staff must be made aware immediately via a page.
(3) Supervise Junior Resident
- Refer to Junior Weekend Call Responsibilities Section for specifics.
Call Skills
Covering the #23305 Pager
- PENDING
- Common Questions:
- Can I rush a specimen?
- Refer to Processing RUSH Specimens section in the Call Skills below.
- Where do I drop-off a specimen?
- Surgical specimens: after hours/weekends, have surgeon/clinician drop it off in the front OR fridge and sign the book
- Difficulty ordering surgical pathology evaluation during EPIC Beaker transition?
- Starting 11/03/2024, the new specimen ordering screens for providers will go live as a part of the transition to Beaker. See below email from Jenn (10/29/2024) with attached powerpoint
- "Starting Sunday, November 3, the new specimen ordering screens for providers will go live as part of our transition to Beaker. This change will be significant for some clinicians, and we anticipate minor ordering issues on Monday. If you receive questions or calls about ordering, please direct them to me or Sarah, and we’ll work to connect them with the onsite EPIC support team. The attached PowerPoint details the changes you will see for AP specimens collected in procedural areas, from ordering to accessioning, and highlights updates to requisition formats."
- PDF with more information (LINK HERE)
- Starting 11/03/2024, the new specimen ordering screens for providers will go live as a part of the transition to Beaker. See below email from Jenn (10/29/2024) with attached powerpoint
- Can I rush a specimen?
Accessioning
- Guides on how to accession a specimen
Frozen Section Cutting
- The histology lab provides a histotech in the frozen lab from 9:00am to 5:30pm Monday through Friday to cut and stain frozen tissue. Outside of this time, it is the frozen section / on-call junior resident's responsibility to cut frozen slides
- If you come across an issue when using the cryostat after hours, you can:
- Troubleshoot the cryostat yourself (if comfortable).
- Walk to the histology lab across the hall and ask for assistance. Many of the histotechs have had experience using a cryostat (even if they are not commonly cutting at MGH) and will be able to help with troubleshooting. Please note that after hours, these staff are unable to cut your frozen slides for you.
- See the following SOP (111736.8998 Cryostat Use Instructions - MGW Surg Path) for some helpful orienting diagrams to the cryostat (LINK HERE)
- Tips:
- Practice, practice, practice: Repetition is key. If you want "practice" tissue, check the placenta fridge for an older specimen that has already been signed out.
- Don’t over-freeze in liquid nitrogen. Typically 12-13 seconds in liquid nitrogen followed by slow freezing in the cryostat works well.
- Breast sentinel nodes are often fatty and thus challenging to cut. Here are some suggestions from our histotechs:
- Orient the specimen such that lymphoid tissue is near an edge of the chuck, rather than in the center with the fat near an edge. This way the blade cuts into lymphoid tissue first, rather than fat first.
- It’s easier to pick up a section when you have a little extra OCT edge to “pull” on.
- When you’re into the specimen, look and confirm that you’re cutting into lymphoid tissue first. If not, re-orient the chuck so that the blade cuts into lymphoid tissue first.
- Keep the blade cold. Use the gauze to drip liquid nitrogen onto the blade. Per the histotechs, this works better than freezing the chuck/specimen.
- If all else fails, try increasing the thickness of the section. The histotechs don’t usually go above 6-7 microns, but sometimes it takes us residents 8 microns to get something on the slide.
Cutting Infectious Cases
After cutting an infectious case (TB, HIV, HepB/C), the cryostat should not be further used before being decontaminated.
- Close the top panel on the cryostat and place the yellow placard on top of the machine (placard says something to the effect "Machine Shut Down for Decontamination").
- Power off the cryostat so that it begins thawing. See photo to right for location of on/off switch.
- Inform histology that cryostat has been powered off and needs to be eventually cleaned.
Loading the Processor
- Step-by-step guide to loading the processor - Guide for Residents from Residents
- The tissue processors run on Saturday and Sunday on a regular weekend. The first time you take call on a weekend, ask the senior resident to help you with loading the processors. If processors are loaded before 5pm, there should be a histotechnologist in the lab who can help you with questions. When loading the processors on weekends, it is necessary to document the cassettes submitted to histology by taking a picture of the slide basket and printing it. This may be done using the specimen camera on Blake 3. Leave the printed picture on top of the processor you have loaded, labeled with date, time, and your initials. You do NOT need to scan the cassettes to the processor.
- Processor #1 (the one behind the door) should not be used as it is the processor for neuro/CJD cases.
- When loading the processors, make sure to pick a processor that is not currently being cleaned, or has low fluids (which will be designated on the main screen).
- Specimens should only be run on 4-hour (small specimens, like biopsies) and 8-hour (large specimens) programs on the weekend. Select either run immediately or set to finish by 3:00am the next day (usually run immediately is the preferred method). If there is a histotech, you can ask them what the best end time is.
- Small biopsy specimens should not go on the 8-hour run as the tissue will become brittle and “overcooked.”
- The histology lab is staffed 7:30 am-4:00 pm on the weekends, and there is often someone coming in at 3:00 am on Sundays, so it is generally safe to set the tissue processor for 7:30 am, but if there is something urgent Saturday night, email William and let him know and he will let you know if there’s 3:00 am staffing on Sunday. The same guidance applies for holidays.
- Histology staff will start at 3:00 am on weekdays.
- If you leave cassettes in formalin over the weekend (I.e. if you had to gross a breast or something) you can bring the container into the histo processor room with a note of when the tissue went into formalin, and it will be loaded when ready.
- Cassettes in decal are checked by histology on Sunday, so you do not need to check them.
- Inquiries about weekend rush cases should be directed to the senior resident on-call.
- Low threshold to call the contact numbers posted in the histo lab, including William Anim (617-962-2793), who is very helpful. There is also an on-call histotech for holidays that can be contacted with any questions or to coordinate rushes.
Processing RUSH Specimens
- Page/Question: Clinician wants to rush a specimen.
- Action: Call the histology lab (x4-1425) and provide them with the case number to request a priority change to make sure the case is processed as a Rush and email the RUSHblock email group <RUSHblock@partnershealthcare.onmicrosoft.com>.
- Note: The case WILL NOT be rushed if the histology lab is not notified. Do NOT change anything in CoPath.
- Page/Question: Clinician wants to rush a cytology specimen.
- Action: Email Ron Arpin <rarpin@mgh.harvard.edu>.
- Page/Question: Clinician wants read of rush case after hours.
- Action: It is likely the specimen was only recently changed to a rush (if it was originally a rush case, it would have been read earlier in the day). Ask the clinician if the patient is stable, and if clinical management would change overnight based on the results. In most situations, the patient will be stable--tell the clinician that you will notify the attending/resident via email, and they will follow-up the next morning.
- During the week: Rushes dropped off before 8pm should be grossed and loaded on the processor. If before 6pm, you can ask a PA or small gross tech to gross the rush.
- Rushes dropped off after 8pm should involve a conversation with the ordering/paging clinician regarding when the next day they need a read and whether the specimen is already in formalin. If they need it first thing in the morning and the specimen is already in formalin, then the on-call resident must go into the hospital to gross and load the specimen on the processor. Otherwise, it is okay to leave the specimen until the next morning, if it is already in formalin. Regardless, the RUSHblock should be emailed after the urgency is discussed with the clinician. Include in the email which clinician you spoke to, by what time they expected a read, and whether a PA needs to gross the specimen in the morning or if the specimen was already grossed and loaded onto the processor.
- For RUSH biopsy specimens received afterhours Sunday through Thursday that require fixation prior to being loaded on to a 4-hour processor, gross them in peach cassettes, email RUSHblock and the corresponding attending, and place the cassettes in the Tupperware labeled “3am processor, 4 hour run” located next to the 3am breast bucket. This Tupperware will be loaded by histology at 3am Mon-Fri morning.
- If you get a large specimen after hours that is a next day rush and has already been fixing in formalin, the specimen should be grossed. Send an email to the RUSHblock, including Jenn Patel, Sarah Ferguson, and Kara Tassinari, and they will ensure the rack gets loaded once a PA gets in the next morning. If the specimen is fresh/hasn’t been prepped and needs to fix for 8 hours, please forward this request to the RUSHblock, Jenn Patel, Sarah Ferguson, and Kara Tassinari, and it will get grossed first thing the next morning.
- Bone Marrow Biopsies that are labeled rush WITHOUT a RUSH page do NOT need to be processed by the on-call residents after the accessioners and PAs/small gross techs leave (~6-7 pm) during the weekdays, the bone marrow biopsies can be left in B+ until the following morning. Bone Marrow Biopsies labeled rush WITH an accompanying RUSH page DO need to be processed by the on-call resident. Please contact the Hematopathology fellow and/or attending on service if there are any questions or if there is a RUSH bone marrow biopsy over the weekend.
- On the weekend: Any rush or clinically urgent cases should be accessioned, dictated, and grossed by the on-call resident. You must load all of the rush specimens unless they come in on Sunday after you have loaded the processors (unless a read first thing Monday morning is required by the clinician).
- Email the RUSHblock group email to alert histology about the rush case. Be sure to include the attending and resident that will receive the case.
- If you are notified of a rush being delivered after you have the loaded the processors on Saturday or Sunday, contact the clinician. If the clinician is insistent that the results of the slides will change clinical management of the patient and that this cannot wait until Monday, first talk to the on-call attending to make sure they are available for a Sunday read. If yes, you must gross the specimen, load it into the processor, and notify the RUSHblock..
- If it can wait until Monday morning, send an email to Jenn Patel, Sarah Ferguson, Derek Kingman, and Kara Tassinari asking them politely to gross the Sunday rush first thing when they get in on Monday morning.
- Always load processors after the specimen has fixed. Even if a processor is set to start later, specimens should be fully fixed before loading to ensure high quality tissue sections.
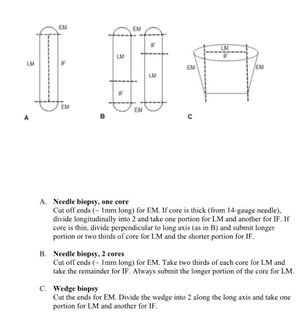
Core Biopsy Handling - General Principles
- If a core biopsy is taken for a focal lesion (e.g., suspected tumor), it may require a significant amount of stains. Preserving tissue in multiple blocks ensures there is enough material for the pathologist to fully evaluate the lesion.
- In general, for FOCAL lesions:
- 2 cores = one in each block
- 3 cores = two and one in each block
- 4 cores = two in each block
- 5 cores = two, two and one in each block
- For detailed specifics, including a helpful subspecialty-specific table, regarding core biopsy allocation, see MediaLab SOP111736.3276 Core Biopsy Fixation and Processing - SURG PATH (LINK HERE).
Processing Top Priority Specimens
- GI biopsies from inpatients are designated “Top Priority” or “Inpatient” on the requisition (handwritten or stamped).
- These specimens should be accessioned, grossed, and loaded onto a processor so they are out by the next business day. Refer to sections below for accessioning & processing guides.
- Weekend - Typically Monday
- Weekday (M-Th) - next day (residents on call during the week should help gross these cases if they see them and the PAs have gone home for the night (typically between 6 - 8 PM). If accessioning staff are still around they should still accession the case.)
- These cases are accessioned as Top Priority and use orange cassettes, but they do not require a RUSH email.
- Residents are not expected to come in overnight to submit these cases. If the on-call residents are busy with other duties (e.g., multiple rushes and frozens), they will email Jenn/Sarah to help arrange grossing the specimen first thing the following morning.
Prepping
General Principles
- Generally, specimens should be inked/measured+weighed/opened, photographed if necessary, placed in formalin, and placed on the shelf under the grossing lists next to the scanner.
- When in doubt, take a photo.
- When in doubt, call your senior.
- Specimens that are routinely inked, should be inked (definitely in areas that are going to be disrupted by opening the specimen and in areas where it may be difficult to reconstruct what was true margin after formalin fixation).
- Any specimen which requires special studies (e.g. a POC requiring cytogenetics) should be accessioned, and material should be taken for special studies.
- Note and/or mark any external findings that may be diagnostically important but more difficult to see when the specimen has been opened and fixed (like small serosal nodules).
- Non-reproducible measurements should be taken.
- This doesn't mean all measurements - just the ones that matter and cannot be reliably performed after your specimen handling/formalin fixation.
- Examples: weight of uterus - yes, length of cervix - no; how far colon tumor is from margin if it is less than 5 cm - yes; if it is really far - no.
- If prepping instructions on AP Call Info page unclear or missing, check specialty-specific grossing manuals.
- Lymphoma workup, including frozen assessment, should be performed if necessary.
- Sometimes there is a PA grossing on the weekend. They are there to help you and you can ask them questions. The calendar for the per diem PA is on the wall to the right of Jenn’s desk.
- If the Junior hasn’t prepped a specimen before, he/she should prep them under direct supervision of the Senior. There are detailed descriptions on how to prep all specimens in the grossing manuals on the Hub; however, it is still best for the Senior to support the Junior and be present with them at the bench when they are learning how to prep. “’Don’t forget to put formalin stickers on all prepped specimens!!”’
- Most small specimens: are already in formalin, just put them on the shelf as they are.
- Most medium sized specimens: gets tossed into formalin except...
- Fat pad biopsies: for amyloid (half frozen for IF and half into formalin for permanents).
- Gallbladders: open before tossing into formalin, be sure to put stones in the jar so grossing staff knows they were there.
- Bowels: Open them before putting them in formalin! Open them before putting them in formalin! Open the ileocecal valve in right hemi-colectomies! Pin out if removed for tumor or suspicious for tumor. Sometimes figuring this out is harder than you think! The pre-existing diagnosis is not infrequently wrong, so use your judgement.
- Heart explants: weigh, photograph front and back, freeze a piece of left ventricle for IF, and put a tiny piece into EM fixative. Put in formalin.
- Don't forget to accession the specimen in order to create an asset label for the frozen component (see instructions under "cardiac biopsies")
- Atrial appendages: Need tissue saved for IF
- Liver explants: weigh, photograph, take the margins (hepatic veins and hilar margins = bile duct, hepatic artery, and portal vein), slice at 0.5 cm intervals, stuff with paper towels to ensure fixation. Put in formalin.
- Lung explants: weigh, photograph and take bronchial and vascular margins. Put in formalin.
- Breast specimens: Do not need to be grossed unless explicitly asked by the breast attending. Breast specimens that are held into the weekend or overnight have been prepped and placed in formalin for the per diem PA to gross or will be grossed the following day by PA staff. Mastectomies: weigh, ink, section through, put in formalin. Reductions: weigh, section through, put in formalin. See specific details below.
- Uteri: Weigh, ink (if appropriate), bivalve and photograph. No need to slice up as you would on frozens, leave it otherwise intact. Put in formalin.
- Cervical cones: Refer to GYN grossing manual. Will need to be opened & pinned prior to fixation
- Kidney: Check if it’s a medical renal or tumor case. If tumor: weigh, ink, take margins and bivalve, then put in formalin. If medical: check the renal path grossing manual (LINK HERE); these commonly need EM & IF allocated.
- Fragmented Leiomyoma: Weigh and add formalin.
- Lipomas: we no longer take a portion of fresh tissue for cytogenetics, no matter the size.
Products of Conception
- Look up the patient history. Often times, the specimen is for “r/o ectopic”. Notify the senior resident. Float the tissue in a saline-filled Petri dish to identify villi (dissecting microscope in the frozen section lab can be helpful).
- If villi are definitively grossly identified, notify the clinician and submit one representative section of the villi.
- If no villi are definitively grossly identified, notify the clinician and submit the entire specimen as a RUSH for permanent evaluation.
- For other types of POCs, contact the clinician to ask what their question is, how urgently the specimen needs to be processed (i.e. over the weekend or Monday morning), and whether additional studies need to be sent (e.g. cytogenetics). You can also loop in the OB attending on-call afterwards via email.
- If you have any request for karyotype (cytogenetics) on a POC or fetal tissue please follow the steps outlined below. 1) Use sterile kit to identify villous or fetal tissue. 2) Put villous/fetal tissue into cytogenetics media tube. 3) Label with Copath label. 4) Print cytogenetics order requisition from EPIC. 5) Write FOR BWH CAMD in bold lettering at the top. 6) Write the sample into the send out logbook 7) Put the sample and a copy of the requisition into the BWH CAMD bin (or in the refrigerator on the weekend).
After-Hours Breast Specimen Prepping
Breast specimens accessioned after-hours (after 6pm) will need to be prepped by the on-call team.
Resources:
- MGH Breast Specimens: On-Call Prepping LINK HERE
- MGH Breast Grossing Flowcharts LINK HERE
- MGH Breast Technical Grossing Manual LINK HERE
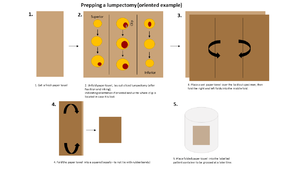
Mastectomies AND lumpectomies should be imaged in the Faxitron (pre-prep), inked, sectioned and put into an adequate amount of formalin. If you have any questions, please ask your senior resident/refer to the breast grossing manual/flow sheet on Learn Pathology. If it’s a final margin, those can just go in formalin. for sentinel lymph nodes, if the case is already accessioned and it is straightforward, it is nice to gross the specimen so IHC can get started. If, however, the case is not accessioned or it is a complex sentinel node dissection, it is OK to put in formalin. You do NOT need to write the "time in" formalin for breast specimens."
When prepping mastectomies, section the specimen from posterior to anterior so that it stays together (posterior surface face up (and anterior surface down on the chuck) along the medial to lateral axis). For lumpectomies, section the specimen entirely through and lay out the "slabs" of tissue on a labelled paper towel and wrap up the specimen (see photo).
Reminder that late cases need to be accessioned in order to image in the Faxitron – this is likely a rare situation for an on-call resident, as the accessioners stay until ~7 pm.
If prepping on-call, initial the container lid of the specimen that you prep – this will allow continuity for questions if they come up by the grosser.
Dermatopathology / Skin Biopsies / Staged Mohs
- Skin biopsies for immunofluorescence
- Biopsies are usually delivered to Pathology in Michel’s transport medium to be processed for immunofluorescence studies. The tissue can be left in Michel’s medium for up to 72 hours (preserves tissue and its antigenicity), so for weekdays and normal weekends, your only job is to make sure the sample is placed in Michel’s medium (do NOT leave it fresh or try to freeze it yourself). The IF lab will process it the next business day.
- If there is a RUSH derm case with an IF component, don't worry about accessioning the IF component. Just accession and gross the part in formalin, and leave a note for the accessioners/email Jenn and Sarah about the IF component to be processed and accessioned the following day. See more specific details about derm IF below.
- If a clinician calls you looking for Michel’s medium and they are unable to obtain it on the clinical side, we have a small stock either in Blake 354 by the accessioner’s window. The medium can be used even if there is salt precipitation around the lid.
- Dermatology is responsible for sending their specimens in Michel’s medium--if they ask you for it, let them know that is their responsibility for future reference (but of course provide it to preserve the current specimen).
- Exception: The ONLY time you should freeze the biopsy yourself is if it’s the Friday afternoon/evening of a holiday/long weekend and it’s possible the tissue will sit in Michel’s medium for longer than 72 hours before it can be frozen by the IF lab. Freeze it in OCT on a chuck (following the same procedure as cardiac biopsies above) and store it in the -80 C freezer for Warren 5.
- Another situation is If you and the clinician are unable to find Michel’s medium. Don’t panic. Michel’s medium is a transport medium, not a fixative. It preserves tissue for IF for up to 5 days but does not actually do anything to “fix” it. In this case, ask that they personally and expeditiously bring the specimen to you in the frozen lab in saline and freeze it once they deliver it to you.
- Check with someone more experience if you are unsure about how to orient the specimen for freezing.
- If a clinician calls you looking for Michel’s medium and they are unable to obtain it on the clinical side, we have a small stock either in Blake 354 by the accessioner’s window. The medium can be used even if there is salt precipitation around the lid.
- Slow / Staged Mohs Excisions:
- Premise: Surgeons will remove skin cancers in “stages”, leaving the resulting skin wound open until the pathologist has confirmed margin status microscopically. The surgeons will send a skin ellipse and separate skin margins that need to be urgently processed to confirm margin status and plan additional surgical interventions.
- Pathology Resident Responsibility: These specimens may arrive in the gross lab after 6:00pm. While on call, gross these specimens for a next day read (if specimen arrives on a Monday-Thursday night) or for a Monday read (if specimen arrives Friday night / Saturday / Sunday)
- Per dermatopathology staff consensus: “Having the slides ready the next day morning is very important so we can obtain deeper sections and rush immunostains if needed. Likely a very rare scenario, but if a slow Mohs or staged excision arrives after 6pm during a weekday, having it grossed that evening and be processed overnight will be best for the patient. For case arrived late on Friday, it does not need to be processed for Saturday. It needs to be grossed and be processed overnight on Sunday so we can have the slides first thing Monday morning.” "It is important that we have the slides for review first thing the next morning."
- How To?
- Refer to Dermatopathology subspecialty grossing manual (LINK HERE). Please read the relevant portion entirely before proceeding. If there are any doubts regarding instructions, please contact the senior resident and/or dermatopathology staff for guidance. This is not a specimen residents gross frequently, so special attention and care is needed.
Uncommon Biopsies
Fat pad biopsies
- Cut it in half, save half for frozen, and put the other half in formalin.
- Page/Question: How big should a fat pad biopsy for amyloid be?
- Answer: Per Dr. Stone, it should be 1.0 cm^3 in size.
- Page/Question: How big should a fat pad biopsy for amyloid be?
Cardiac biopsies
- Usually received on ice. Do NOT use a chuck to freeze it. Write the patient information (Name, MRN, Accession Number) on the back of a biopsy card. Freeze by putting a drop of OCT on a biopsy card or directly into the cryostat and embed the cardiac tissue within it. After the drop freezes, attach the CoPath container label to the paper (see below), and put it into a small bag with a copy of the requisition form. Scan the Asset label to “SPUF” (SPU Freezer for Frozen Saved Tissue) and put the asset label in the bag. Place it in the -80C fridge for Warren 5.
- How to accession an Asset (create an Asset label)
- FYI: Using a chuck creates a plane between the chuck OCT and the OCT added on top, which creates processing issues.
- Note: There should be another portion of the specimen for permanents (which is usually accessioned and handled by the accessioners during the day). After hours and over the weekend, if you cannot find another portion for permanents, check with the clinical team before freezing the tissue.
- After hours or weekend/holiday endomyocardial biopsies for transplant rejection: Per JST, the on call resident should come in after hours to freeze the piece for IF. Rush endomyocardial biopsies accessioned over the weekend are for a Monday read (or next business morning if a holiday weekend).
- Apical core biopsy (received fresh, not on ice):
- Accession the part type as "Heart Exc Left Ventricle" (so it'll be properly processed; using “heart biopsy” generates a different protocol)
- Save a piece frozen (don't forget the asset label; see below)
- Save a piece for EM.
- Bottom line: If there is a fresh heart specimen (apical core, ventricle biopsy, etc.) in the fridge --> accession/create asset label and save portion for EM AND IF.
Muscle biopsies
- As soon as you hear that a muscle biopsy is coming, contact the SPU manager (if during regular hours)
- If after hours, use the on-call tech list (posted on the inside of the SPU door on Blake 3) to call the first on-call tech and ask if someone from SPU will be in/can come in to process the muscle.
- If no SPU staff are available, you will process the muscle.
- Call your senior, use Javier's instructional video (LINK HERE), or reference the 10-step guide to Muscle Biopsy on Media Lab (LINK HERE) for help.
- Remember to save a piece of muscle for electron microscopy and to enter a gross in CoPath: "Received labeled, measurements, portion sent for EM and remaining to SPU"
- Once complete, send an email to SPU staff at MGHIHC-SPUStaff@partnershealthcare.onmicrosoft.com and RUSHblock to let them know about the case.
- If this happens, notify the chief so that we can keep track of how often this is occurring.
Temporal artery biopsies
- Any temporal artery specimen (regardless of whether or not marked RUSH) should be treated as a RUSH and grossed/processed right away.
- Do not cut them. Wrap each temporal artery in histowrap, place in a peach cassette with a ribbon, and write “temporal artery” in pencil on the side of the cassette.
- If a frozen section is requested to confirm if artery is present, shave the two ends of the fragment and freeze the cross-sections. Temporal arteries from Mass Eye and Ear accessioned to Eye Path as Rushes on Fridays, in recent experience, have been treated as Monday rushes, per Eye Path request (ie, they are not re-accessioned to CV for Saturday read). Always a good idea to check in with Eye Path just to confirm though.
Rush kidney biopsy for transplant rejection
- During work hours, IR-guided kidney biopsies for transplant rejection are checked by a histo tech for adequate glomeruli at the time of biopsy. After hours and on weekends/holidays, the senior resident on call should assess these biopsies for adequacy (at least 7-10 glomeruli seen using the dissecting microscope).
Medical renal biopsies after hours
- More information is located here; also see image embedded on the right-hand side of this page for more details.
Liver cores after hours
- As the on-call resident, you may get questions from accessioners regarding how to accession liver core biopsies when the PAs are unavailable. These biopsies need to be accessioned as 1. Focal, 2. Non-focal, or 3. Transplant. It's important for them to make this distinction so the right stains are ordered up front!
- While it's always good to confirm in Epic the clinical situation/procedure note, as a broad overview:
- Focal biopsies are biopsies targeting specific lesions (e.g. tumors).
- Non-focal biopsies are assessing non-specific conditions (e.g. non-specific LFT elevations, inflammatory diseases, etc.)
- If s/p liver transplant (should be in Epic/procedure note).
Transbronchial Biopsies
- Starting 08/2024, we will start receiving lung TRANSPLANT/ALLOGRAFT transbronchial biopsies for R/O rejection in a SINGLE container!! The same areas and general number of tissues will be sampled and submitted all as one part. The accessioning will remain the same but please add a second block to the protocol before saving. If any additional parts are received please follow the same protocol for each.
- Please use the following format for grossing:
- A1 - maximum of 4 tissues
- A2 - remaining tissues
- All tranplant lung biopsies are accessioned as TOP PRIORITY unless indicated as RUSH by the submitting provider.
Uncommon / Unique Frozen Section Scenarios
Rapid Molecular (Neuro / Pulm Cases) & Cutting Unstained Slides
- This applies to lung and neuro rapid molecular cases. The CID lab is expanding the catalogue of rapid molecular testing they are able to perform. They have requested additional slides so they have sufficient material to workup these extra tests. The workflow will not change significantly except for the pathologist should clearly indicate to the histotech how many slides to cut after reviewing the frozen slide. If there is sufficient material, please increase to 19 unstained + 1 H&E. If there is scant tissue, revert to 9 unstained + 1 H&E. It is okay to cut 19 unstained sections with two sections per slide. Place cut slides in ISOPROPONOL(not ethanol like normal frozens) before placing them to air dry in a tray). The isoproponol can be found next to the cryostat in a glass jar on the shelf. See photo.
- During weekday work hours, the histotech in the frozen section lab will cut the slides and then page Rapid Molecular (29059). After hours and on the weekend, the on-call team needs to cut the sections as outlined above. The unstained slides and the H&E recut (not the H&E used for frozen section diagnosis) should be placed with a copy of the requisition on the window shelf between the six-headed scope and the accessioning computer in the frozen section lab. Please keep the original paperwork with the specimen. An email must be sent to Nick Jessop at NJESSOP@PARTNERS.ORG indicating that there are slides for molecular pickup the following business day morning. In addition to the email, you can still page Rapid Molecular as they check the pager first thing Monday morning, but molecular staff are not available during the weekend.
- The slides do not require refrigeration. They can be left at room temperature until pickup the following business day morning.
Ultra-Rapid Molecular for Pulmonary Pathology Primaries/Metastases
- In Frozens, you will receive a specimen in saline from either the OR directly or a cyto tech that has something on the req about ultra rapid molecular testing (usually primary PP case or metastatic pulm case)
- All ultra rapid molecular cases are automatically accessioned as a RUSH (vs routine rapid molecular)
- CALL THE PULM ATTENDING!!!
- Confirm with attending how much to freeze, then treat it like a normal frozen (even if FZN is not checked as a YES on the req – it is implied that a frozen section will be done in order to get slides for molecular testing)
- Once the frozen section attending has confirmed that there is lesional tissue, then attending will decide how much to cut for molecular
- If you have extra tissue, process the frozen remnant per usual (A1)
- Ask PP attending if they would like blanks ordered up front or IHC (e.g., ALKTR and ROS1)
Transplant Cases
- Page/Question: Organ transplant people page you with a heads-up about a prospective transplant
- Action: Ask when the transplant is happening and ask where the team is now (Ask for the OR number, which will make it clear if they have an OR yet or not, and ask if donor is in OR). Tell them to page you again ~30min prior to the actual procedure. Notify them of our frozen policy between 8pm and 8am, that we will not be in house and need advance notice.
- Note: The transplant could happen at any time after they first notify you (or not during your shift, or not at all), so to minimize the waste of your time, ask for a more timely page (which may still involve a long wait, but it’s the best option). No matter when they tell you the transplant is happening, it will almost certainly be later than that. That said, if they say the frozen is in 1 hr and they have the OR, it’s best to stick around and be ready.
- When you cut the frozen, cut 2 H&E slides. The organ bank will bring a worksheet for the senior resident to fill out (re: histology of the organ). When they are done, they take one of the frozen slides with them, so in order for us to have an H&E on file, you should cut a second one at the time of the frozen.
- Evaluation and handling of liver biopsies:
- Freeze the core entirely.
- Cut TWO slides for each specimen, as one slide will go to UNOS.
- The most important thing to evaluate is the percentage of macrosteatosis, as this correlates with post-transplant rejection (they like to see <30%).
- Complete the UNOS form, which includes additional details such as % macrosteatosis, % microsteatosis, extent of lobular/portal inflammation, fibrosis, and necrosis.
- Retain a copy of the UNOS form for MGH records.
- Label the UNOS slides with the patient’s name, UNOS number, and specimen type (e.g. right lobe of liver).
- Leave slides and paperwork at the frozen lab desk for proper accessioning in the morning (do not accession yourself). Leave a note/e-mail accessioning to inform them.
- Take the tissue down like a normal frozen and put in formalin.
- References:
- Evaluation and handling of renal allograft biopsies:
- Freeze the core entirely.
- Cut TWO slides for each specimen, as one slide will go to UNOS.
- The most important thing to evaluate is the percentage of glomerulosclerosis (i.e. the number of sclerosed glomeruli vs. total glomeruli).
- Note: They need 50 glomeruli evaluated at a minimum, so if you don’t count 50, get a deeper section and add the numbers together.
- Complete the UNOS form, which includes additional details such as extent of glomerulosclerosis, presence/extent of interstitial fibrosis, tubular atrophy, and arteriosclerosis/hyalinosis.
- Retain a copy of the UNOS form for MGH records.
- Label the UNOS slides with the patient’s name, UNOS number, and specimen type (e.g. right lobe of liver).
- Leave slides and paperwork at the frozen lab desk for proper accessioning in the morning (do not accession yourself). Leave a note/e-mail accessioning to inform them.
- Take the tissue down like a normal frozen and put in formalin.
- References:
Lymphoma Work-up
- In general, if there is sufficient tissue for multiple cassettes, perform a frozen section to triage the material. If you feel that the specimen is too small, page the HP fellow or attending on-call and ask how to handle the specimen.
- See this very helpful information sheet posted above the frozen lab bench.
- Often times, a specimen will come with a requisition that says “r/o lymphoma” but no callback is requested. In these cases, still follow the above guidelines and perform a frozen section to triage the tissue if enough material is available.
- Eye pathology samples (usually from MEEI) labeled "orbital mass" and received in the frozen section lab in saline should be sent for flow.
- Usually two parts are sent for "orbital mass" samples, a larger piece in formalin for permanent and a smaller piece in saline for flow cytometry; please confirm receipt of these two parts (by looking in EPIC or discussing with the surgeon/eye pathologist)
- The smaller piece received in saline can be sent directly for flow cytometry without performing a frozen section; discuss with eye pathologist on call if any questions arise
- Flow Cytometry Label Reminder: Please double check that the correct label is placed on all flow send out tubes. It MUST be the container label that indicates the part type (A, B, C, ...) of the specimen; it CANNOT be the requisition label.
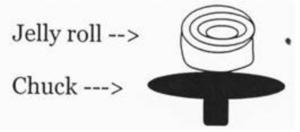
Skin “Derm jelly roll - rule out Steven Johnson Syndrome”
- At night or on the weekend, you may rarely get a skin “jelly roll” with the clinical history of “r/o SJS/TEN vs. staph scalded skin syndrome (SSSS).” Basically this is a rolled up portion of sloughed skin that should be cut like this in order to evaluate the extent of involvement of the epidermis (the jelly roll should show acantholysis of the epidermis). In general, punch biopsies are NOT typically frozen for this purpose, and you should ask the clinical team if they instead would prefer to have punch biopsy specimen rushed.
Children's Oncology Group (COG) Specimens
- Refer to working document "Guidance for Pediatric and Perinatal Specimen Handling" for more specifics (LINK HERE)
- The Children's Oncology Group (COG) runs clinical and research assays on many types of childhood cancers. These assays are often required to enroll patients in front-line clinically actionable treatment. As such, tissue triaging (to include possible frozen section evaluation) for "COG banking" is a part of standard clinical care
- The MGH COG team is contacted about incoming patient samples (COG@massgeneralbrigham.org - includes Dr. Kate Dannheim and other grossing room staff). At this time, additional pathology team member (namely, on-call frozen section or overnight residents) will be looped in to discuss timeline and triaging plan.
- If questions arise:
- Refer to above document for COG recommendations for tissue quantities.
- Contact Kate Dannheim (pediatric pathology) or appropriate subspecialty (hemepath, GU, GI, etc) as needed for questions or guidance
- Please provide feedback as desired to Kate Dannheim / MGH AP chief resident
General Hematopathology Resources
Bone Marrow Biopsies
After-Hours Rush Bone Marrows
NON-PAGED Rush Bone Marrows After 6-ish: Please be aware that for NON-PAGED rush bone marrows received after 6 PM, it is acceptable to leave them for the next morning. There is no need for anyone to stay late or to request on-call residents to perform grossing. These specimens are approved for grossing in the morning and should be submitted on the first processor of the day.
PAGED Rush Bone Marrows – No Specific Cutoff: Any rush bone marrow with an accompanying PAGE should be grossed and processed on the same day, within reason. Due to their increased clinical urgency, the expectation is to have these cases grossed and processed on the same day to facilitate early morning slide delivery.
- More details on grossing a bone marrow biopsy are listed in the Hemepath technical grossing manual
- Be sure to check if it was a dry tap (second core received in saline jar) as there is a different processing procedure.
How to know if there was a rush page for a bone marrow?
The RUSH pager is clipped to a binder in the accessioning area over the large copier. Every page is logged with the Date/Time, Name, MRN, type of sample (Peripheral blood or BM) with an area to sign when the specimen is received in the lab. Please sign the logbook if you accession a rush page bone marrow after hours.
Helpful Medialab Links:
Flow Cytometry / RUSH Leukemia Cases
- In general, ONLY specimens that are accompanied with a RUSH page (specifically, r/o acute leukemia patients) are sent to BWH's Flow Lab. The clinical team knows to send a group page to the Leukemia Pathology Team in these cases (Group #549). ALL other cases, including cases labelled "RUSH" ONLY on the req, have their Flow sent routinely to Mayo. However, the corresponding core biopsy will be elevated from Top priority to RUSH and the aspirate is stained as a rush by special heme (always coordinate with the on-call HP fellow/attending regarding if the core is a same day/next day/next weekday RUSH).
- Regardless if "rushed" via the page or on the req, Myeloma flow samples are ALL sent to Mayo for a combined flow/FISH test (BWH does not do this testing).
Weekday/Routine Flow to Mayo
- An eraser-sized piece of tissue should be placed in Hanks and left in surg path with an accessioner to triage to Mayo via the Core Sendout lab. These should not be placed in the flow bin since they are no longer done on site.
- When sending flow to BWH for a RUSH specimen, place an eraser sized piece of tissue in Hanks.
Weekend RUSH leukemia/lymphoma flow to BWH
- On weekends, the Flow Lab Specimen pager will be forwarded to Hemepath pager (usually covered by a Hemepath fellow). If there is no Hemepath fellow on service, the Hemepath pager will be forwarded to the AP 1st call pager, 23305.
- MGH Hemepath on-call attending will determine priority for RUSH flow testing at BWH.
- Specimens received after 3 PM on Friday up until 11 AM on Saturday can be sent to BWH on Saturday for same-day processing.
- Specimens received after 11 AM on Saturday up until 11 AM on Sunday may be sent to BWH Flow Lab for same-day processing. If it won’t change clinical management to wait until Monday morning, then the sample can be held at MGH to be run as a RUSH on Monday morning.
- Specimens received after 11 AM on Sunday should remain at MGH to be run as a RUSH on Monday morning.
- Once the MGH Hemepath attending determines that RUSH flow testing at BWH is needed, the tissue must be placed in HANKS (or Saline if no Hanks is available), and someone from the on-call team needs to contact Core Lab at 617-726-3637 and request that a STAT courier be called to pick up the sample from MGH for delivery to the BWH * * Flow Cytometry Laboratory. If molecular/cytogenetics samples have also been received on the same patient, those should be sent via the same courier for delivery to BWH CAMD. Core Lab will separate and label the samples individually for delivery to the BWH Flow Lab and BWH CAMD, but you should print an additional CoPath specimen label and include it in the specimen bag (not affixed to anything). Sample must arrive at BWH Flow Lab by 2 PM for same day processing and results. Contact number for BWH Flow Cytometry Lab to confirm receipt: 617-732-5846.
- When sending a rush flow case over to BWH on a weekend (following discussion with and approval by the hemepath attending on-call), you should simultaneously send an email to BWHFlowCytometry bwhflowcytometry@partners.org and BWH LCO Client Services BWHLCOClientServices@partners.org.
- Cases will be accessioned in BWH PowerPath, will cross to Epic and should be viewable by both institutions.
- Cases will be interpreted and signed out by the BWH attending pathologist. Flow plots can be sent to MGH at a later date if they are needed for MRD reference.
- HOLIDAYS other than Sat/Sun: BWH does not staff flow cytometry on holidays, but they try to have someone trained in flow in the main hematology lab on holidays in case of emergencies. If there is no flow person available for the holiday, one of the flow techs will be on call. They do not carry a pager. Instead, call the main hematology lab (617-732-6850) to coordinate the RUSH testing.
- For organ transplant T cell monitoring- redirect questions to Dr. Preffer (p23319)
Helpful Medialab Links:
After-hours lymphoma workup specimens
This section generally applies to specimens accessioned to the HP-B service. For processing of bone marrows, please refer to section above on bone marrows.
- After the on-call team has done a lymphoma workup (if and as appropriate, with input from Hemepath fellow or attending as needed):
- B-plus fixed tissue for both rush and top priority cases should be submitted same day.
- Other specimens designated routine (such as normal-appearing tonsils and reactive lymph nodes) can wait until the next morning unless the senior on-call feels it should be loaded same-day.
- Histology-related notes
- Late night specimens (including those in B plus) can be left on top of the rack that is being loaded at 3 am. We just ask that you pay attention to the following reminders:
- Please make sure that the specimens are fixing for the appropriate amount of time based on specimen size and fixative type – you’ll have to use your judgment here!
- Please make sure that anything that is left there is already grossed and in cassettes. Large pieces of lymph node being fixed in B-plus should remain in Blake 3 for the morning staff to process. If the B plus portion is being sent on the 3 am processor and the formalin portion is remaining in Blake 3, please makes sure that there is a (partial) gross description entered with documentation of the B plus cassettes.
- If a specimen is a rush, please email the RushBlock and let them know if a rush block is with the 3 am run.
- If a HP specimen is split into B plus and formalin, remember that B plus is a rapid fixative, so if you are only going to process a portion of the specimen, it makes more sense to expedite the B plus portion rather than the formalin portion.
Courier Instructions
- Current instructions are posted on the bulletin board above the middle accessioning station and a photo is attached
- Call Skycom courier as soon as you are aware that they will be needed
- Phone: 617-782-1130
- Account: A2217
- Cost Center: 1200-952600-MG-7524
- Destination address: 75 Francis St, Boston MA; Shapiro Bldg 5th floor rm 032
- After calling the courier page cytogenetics and call them at 1-857-307-1500
- CAMD is in house Saturdays and holidays 8am-4pm.
Cytogenetics
- Weekdays:
- Last pick-up around 5:30 pm; prior to this time, give to one of the accessioners (labeled tube, completed cytogenetics form, and photocopy of requisition sheet.)
- After 5:30pm, the specimen can be left in the fridge overnight (prepared as above), and the accessioners will take care of it in the morning (leave a note to let them know it is there!).
- Weekend cytogenetics policy:
- Special courier transport of cytogenetics specimens to BWH CAMD over the weekend should only be performed for the following clinical circumstances: 1) r/o acute promyelocytic leukemia (APML or APL) or 2) r/o Burkitt lymphoma. If the history is not clear based on the requisition and chart, please page the clinician, HP fellow or attending on-call for clarification.
- RUSH cytogenetics for heme malignancies is typically sent from a bone marrow or peripheral blood specimen, but the workflow is currently being expanded to include other types of specimens (including other fluids).
- The core lab typically transports cytogenetics specimens to CAMD. However, if they cannot do it, you must arrange a courier to transport the specimen (see below).
- The cytogenetics fellow on call should be contacted regarding any case that is sent to BWH CAMD over the weekend (pager number 30527). Please also alert the CAMD lab to the RUSH status of the specimen by emailing BWHCAMDAccessioning@partners.org.
- All other specimens are to be kept at MGH for delivery to BWH CAMD on Monday morning. If it is a 3-day weekend, the specimens will be delivered on Tuesday. Specimens should be kept in the refrigerator over the weekend.
- Special courier transport of cytogenetics specimens to BWH CAMD over the weekend should only be performed for the following clinical circumstances: 1) r/o acute promyelocytic leukemia (APML or APL) or 2) r/o Burkitt lymphoma. If the history is not clear based on the requisition and chart, please page the clinician, HP fellow or attending on-call for clarification.
The cytogenetics fellow on call should be contacted regarding any case that is sent to BWH CAMD over the weekend (pager number 30527). Please also alert the CAMD lab to the RUSH status of the specimen by emailing BWHCAMDAccessioning@partners.org.
All other specimens are to be kept at MGH for delivery to BWH CAMD on Monday morning. If it is a 3-day weekend, the specimens will be delivered on Tuesday. Specimens should be kept in the refrigerator over the weekend.
Cytology After Hours: ROSE, Accessioning, WRN1 refrigerators, and Processing
- Please refer to the following SOPs & videos for protocolized information:
- 111736.2419 FNA Cytopathology Procedures and Policies CYT (MediabLab Link here) - scroll to final page for "After Hours Policy"
- 111736.10727 Protocol for Late-Afternoon or Friday RUSH Specimen Request (MediaLab Link here)
- Refer to the following video (~6 minutes) (link here) for guidance on accessioning cytology specimens and processing FNAs placed in ThinPrep preservative or CytoLyte solution; this video is referenced in 3e (page 2) of the SOP above.
- On-call cytopathology physicians:
- Staff cytopathologist: Weekday - Cyto B attending, Weekend - QGenda/LearnPath schedule (QGenda LINK HERE)
- Staff cytopathology fellow (weeknights until 7:30pm): fellow on Cyto B > fellow on Cyto A > fellow on Cyto C
- Potential on-call scenarios (refer to SOPs):
- After-hours ROSE (after 5:00pm)
- Generally, unstained slides will arrive to the Blake 354 Grossing Lab; the on-call resident will stain these slides using the standard frozen section staining protocol. On-call residents are not responsible for picking up the specimen from the OR or for smearing the slide.
- Interpretation: Once credentialed (typically completed during Frozen Section Senior conferences in the fall/winter), on-call senior residents can independently provide ROSE interpretation for clinicians (similar to what is done at MGH with overnight frozen section interpretations). If back-up is desired, the on-call resident can contact the on-call cytopathology fellow (until 7:30pm on weeknights) or the on-call cytopathology staff (any other time after hours).
- Upgrading a previously accessioned cytology specimen (that has a concurrent tissue core) to RUSH
- Occasionally, a tissue core subsequently upgraded to RUSH will have been stored for the weekend/weeknight in a cytology refrigerator (Warren First Floor) with the concurrent cytology fluid. These refrigerators are locked.
- Refrigerator location: From the Warren elevators on the first floor, badge into the WRN 1 hallway. The cytology refrigerators are in the hallway immediately to the left.
- Key location:
- From the Warren elevators on the first floor, badge into the WRN 1 hallway.
- Badge into the first door on the left (WRN113, immediately before the pair of refrigerators).
- The keys are hanging on the wall immediately to the right of the door above eye-level in an envelope. The refrigerator key is labeled #1 or #2 with a black binder clip (each key opens both Refrigerators #1 and #2).
- Specimen location: Specimens that intended to be processed the next day are placed in Refrigerator #2, third shelf from the top ("middle" shelf); look here first when searching for a specimen in these refrigerators.
- Occasionally, a tissue core subsequently upgraded to RUSH will have been stored for the weekend/weeknight in a cytology refrigerator (Warren First Floor) with the concurrent cytology fluid. These refrigerators are locked.
- Specimen processing with ThinPrep 2000 (anticipated very rare scenario)
- Refer to protocol 111736.10727 (MediabLab Link here) for scenarios this would be necessary (item #3 on page 2).
- Refer to the instructional video (link here) for a reminder on using the ThinPrep 2000.
- After-hours ROSE (after 5:00pm)
Autopsy After Hours: Request for Cases, NEOB inquiries, etc
- Occasionally, we are paged to answer questions about getting autopsy permission. You should be able to answer most of the questions concerning obtaining permission after a week on the autopsy service. If there are any questions you cannot answer, contact the senior resident on-call or the autopsy attending on-call. The inpatient floors should all have copies of the autopsy permission form.
- If you are notified about an autopsy that is coming, email the details to the autopsy attending, the Autopsy PA Group, Autopsy Staff Group, and the resident on autopsy who will be getting the case.
- Occasionally, we are paged to answer questions regarding body transport and delivery. It is the responsibility of whoever is transporting the body to us to arrange body transport via a funeral home. If there are questions regarding directions to the hospital, where to drop off the body, or needing access to the morgue, the person can be directed directly to MGH security (617-726-2121).
- If New England Organ Bank (NEOB) pages you overnight to request use of the morgue, tell them that is okay and direct them to security at (617) 726-2121 to open the door. You should also notify the Autopsy team. If the autopsy is pending an MGH autopsy (check admitting ext 63384) and guidance is needed about possible restrictions on specific organs, call Justin Susterich.
- We no longer perform weekend clinical autopsies effective April 1 2023. Per Justin, please note that pages/communication about outside cases coming to MGH over the weekend from affiliate hospitals will be directed to the on-call resident same as weekday after hours. These hospitals do their own consent and will send the decedent over automatically. All the on-call resident needs to do is acquire the clinical information and questions for autopsy from the paging pathologist/clinician and email Monday’s autopsy team the information. If we receive a call about requesting an outside autopsy from a family, then the decedent affairs/techs would get that call and coordinate an email. Claudia and Ariana will still be here on the weekends grossing/accessioning and can help residents if they have any questions about autopsy-related pages that they receive. You should not receive a request for performing a weekend consent with this new system, but if you do, you can defer to Monday’s team.
Banking Tissue for Research
- If you receive a request to bank tissue for research after hours or on the weekend, please politely explain to the person requesting that we are only able to bank during the hours of 7AM-6:30PM Monday to Friday and under the supervision of a PA and/or attending pathologist. No banking of research specimens on the weekend unless a PA happens to be present or collaborating pathologist is available (in either case, “’RESIDENT DOES NOT BANK ON WEEKEND”’). Residents can also reassure the requestor that tissue will remain fresh (i.e. not placed in formalin) in the fridge for supervised collection to occur the morning of the following business day and offer them that option. If you get pushback, feel free to tell them they can contact Dr. Aliyah Sohani as Director of the Lab to discuss further.
- The on-call team will not bank or provide tissue to research teams after hours Monday to Friday or over the weekend.
- Please refer any onerous requests to the program directors and Aliyah Sohani.
- Neuro Tissue for Research
- After 5pm, the senior on call is responsible for releasing neurologic tissue for research requests. All questions should be directed to NP staff and not to fellows.
- First, before any tissue is released, there must be a frozen section diagnosis obtained for the specimen. If none has been performed then a portion of the tissue being submitted for permanent histology should be frozen to confirm the diagnosis.
- For all cases, the senior resident should assess if the amount of tissue being submitted for permanents and research is a reasonable amount. In general, we always want to keep more tissue for permanents than is given away for research. If it seems unreasonable then I have taken some tissue out of the research container and added it to the permanent container (trying to be as sterile as possible if sterility is needed for research). I feel most comfortable when we are giving away only 10-20% of the tissue, though more can be ok if there is a lot in both containers and we will have plenty to make a diagnosis.
- If those conditions are met, cases that can be released by the senior resident without consulting the neuropathology attending on service that day include:
- Schwannoma
- Pituitary adenoma
- Meningioma
- Glioblastoma or a Grade IV astrocytoma that has definitive high-grade features on frozen (necrosis and/or microvascular proliferation)
- Recurrent/residual glioma that shows grade 4 features (necrosis and/or microvascular proliferation)
- Metastases
- Cases that should always be discussed with the neuropathology attending who saw the case in the frozen lab include:
- Low-grade gliomas
- High-grade gliomas that don’t have features to warrant being called GBM or grade 4 on frozen
- Anything else not listed here
- The general principal is we don’t want to give away too much tissue on a case that the grade could change if we see certain diagnostic features by examining more tissue.
- Also, if there is no researcher present to collect the tissue, please make every attempt to contact them and let them know it is available to pick up. Leave the research portion in the fridge next to accessioning and make sure the PAs know to look if the specimen is still there in the morning. If it is not picked up by the morning then the PAs should add it to the permanent tissue submitted. Please send an email to the neuro fellow that a piece was left in the fridge for a researcher to pick. NO page necessary.
- MEE specimen banking after hours
- These are typically handled at MEE by the HN1 attending during the day. In the rare instance that the surgery was delayed and the MEE frozen lab has closed, bankers will come to MGH to collect the research tissue.
- Primary site tumor: bank a small piece (<0.5 cm) of central tumor that does not interfere with depth of invasion or margin.
- Lymph node metastasis: bank a small piece (<0.5 cm) if it is a large metastasis (>1 cm).
- Do not give away tissue that would obstruct the diagnosis. If size/location of the tumor is questionable for banking, contact the HN1 attending (or HN subspecialty head Peter Sadow as backup).
- Sometimes the research tissue is already allocated into a separate container. In this case, you only need to check that the tissue for permanents looks like a reasonable amount for diagnosis.
- These are typically handled at MEE by the HN1 attending during the day. In the rare instance that the surgery was delayed and the MEE frozen lab has closed, bankers will come to MGH to collect the research tissue.