Difference between revisions of "Atypical Lobular Hyperplasia (ALH)"
| Line 1: | Line 1: | ||
| − | __NOCACHE__ | + | __NOCACHE__ |
{{:TOC}} | {{:TOC}} | ||
| − | == | + | <br> |
| − | + | == Introduction == | |
| − | + | {{dxintronodisc|Lobular Neoplasia: Atypical Lobular Hyperplasia (ALH)|Lobular neoplasia represents a neoplastic proliferation of atypical, dishesive, nonpolarized epithelial cells growing in apparently discontinuous, scattered foci within the glandular tree. Pathologists divide lobular neoplasia into two categories based on the number of the neoplastic cells. Lobular carcinoma in-situ constitutes the more florid proliferation and atypical lobular hyperplasia (ALH) the less pronounced version. The diagnosis of lobular carcinoma in-situ requires characteristic neoplastic cells to completely fill, distort, and distend at least 50% of the acini in a single lobule. Lesser involvement merits the diagnosis of atypical lobular hyperplasia.|Atypical lobular hyperplasia does not produce clinical signs or symptoms, nor does it produce calcifications. It is an incidental finding in specimens removed for unrelated clinical indications. Atypical lobular hyperplasia frequently co-exists with other atypical mammary epithelial proliferations and may have the same risk for the development of invasive carcinoma as LCIS. (Clinical follow-up studies have not consistently demonstrated differences in the risks for the development of invasive carcinoma associated with ALH and LCIS.)|None|Atypical, non-polarized, dishesive epithelial cells mingle with indigenous luminal, myoepithelial, and basal cells in acini, terminal ductules, and ducts.|Lobular carcinoma in-situ (greater degree of proliferation), pagetoid DCIS (more cohesive, polarized cells, positive E-cadherin), prominent clear basal cells (polarized, cohesive cells), immature ductal hyperplasia (cohesive, blends with conventional usual ductal hyperplasia)||21-1 Scattered_cells.jpg|The spectrum of ALH extends from cases in which the features of lobular neoplasia are first identifiable to cases that nearly meet criteria for lobular carcinoma in-situ.}} | |
| − | |||
| − | The diagnosis of lobular carcinoma in-situ requires characteristic neoplastic cells to completely fill, distort, and distend at least 50% of the acini in a single lobule. Lesser involvement merits the diagnosis of atypical lobular hyperplasia. | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
The neoplastic cells that compose atypical lobular hyperplasia appear identical to those that make up lobular carcinoma in-situ. Only the number of the neoplastic cells differentiates the two varieties of lobular neoplasia. | The neoplastic cells that compose atypical lobular hyperplasia appear identical to those that make up lobular carcinoma in-situ. Only the number of the neoplastic cells differentiates the two varieties of lobular neoplasia. | ||
{{img4|21-2 ALH.jpg|21-3 LCIS-2.jpg|Atypical lobular hyperplasia|Lobular carcinoma in-situ}} | {{img4|21-2 ALH.jpg|21-3 LCIS-2.jpg|Atypical lobular hyperplasia|Lobular carcinoma in-situ}} | ||
Revision as of 08:24, July 17, 2020
Contents
Introduction
|
Definition: Lobular neoplasia represents a neoplastic proliferation of atypical, dishesive, nonpolarized epithelial cells growing in apparently discontinuous, scattered foci within the glandular tree. Pathologists divide lobular neoplasia into two categories based on the number of the neoplastic cells. Lobular carcinoma in-situ constitutes the more florid proliferation and atypical lobular hyperplasia (ALH) the less pronounced version. The diagnosis of lobular carcinoma in-situ requires characteristic neoplastic cells to completely fill, distort, and distend at least 50% of the acini in a single lobule. Lesser involvement merits the diagnosis of atypical lobular hyperplasia. Clinical Significance: Atypical lobular hyperplasia does not produce clinical signs or symptoms, nor does it produce calcifications. It is an incidental finding in specimens removed for unrelated clinical indications. Atypical lobular hyperplasia frequently co-exists with other atypical mammary epithelial proliferations and may have the same risk for the development of invasive carcinoma as LCIS. (Clinical follow-up studies have not consistently demonstrated differences in the risks for the development of invasive carcinoma associated with ALH and LCIS.) Gross Findings: None Microscopic Findings: Atypical, non-polarized, dishesive epithelial cells mingle with indigenous luminal, myoepithelial, and basal cells in acini, terminal ductules, and ducts. Differential Diagnosis: Lobular carcinoma in-situ (greater degree of proliferation), pagetoid DCIS (more cohesive, polarized cells, positive E-cadherin), prominent clear basal cells (polarized, cohesive cells), immature ductal hyperplasia (cohesive, blends with conventional usual ductal hyperplasia) |
 |
The neoplastic cells that compose atypical lobular hyperplasia appear identical to those that make up lobular carcinoma in-situ. Only the number of the neoplastic cells differentiates the two varieties of lobular neoplasia.
 |
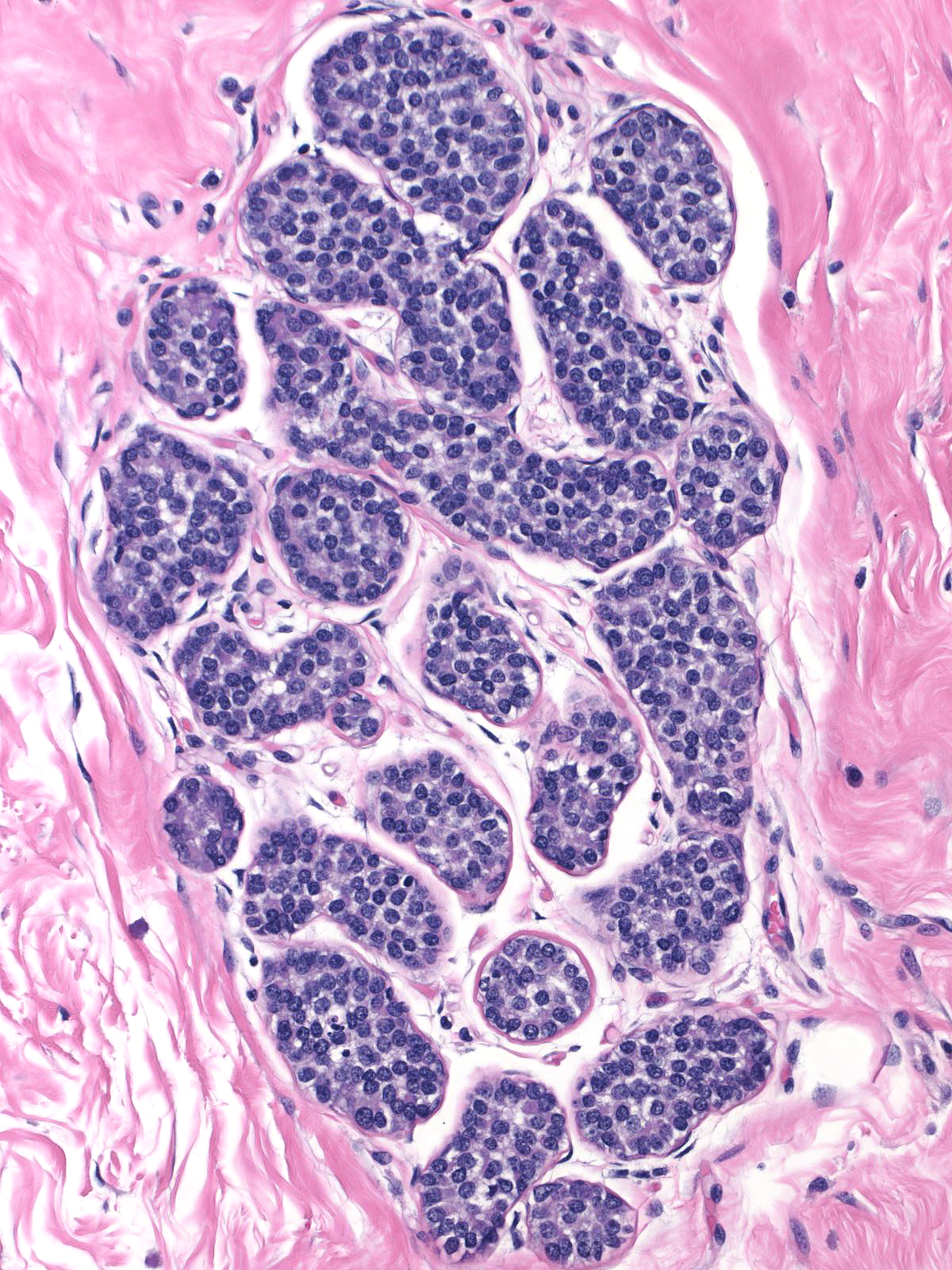 |
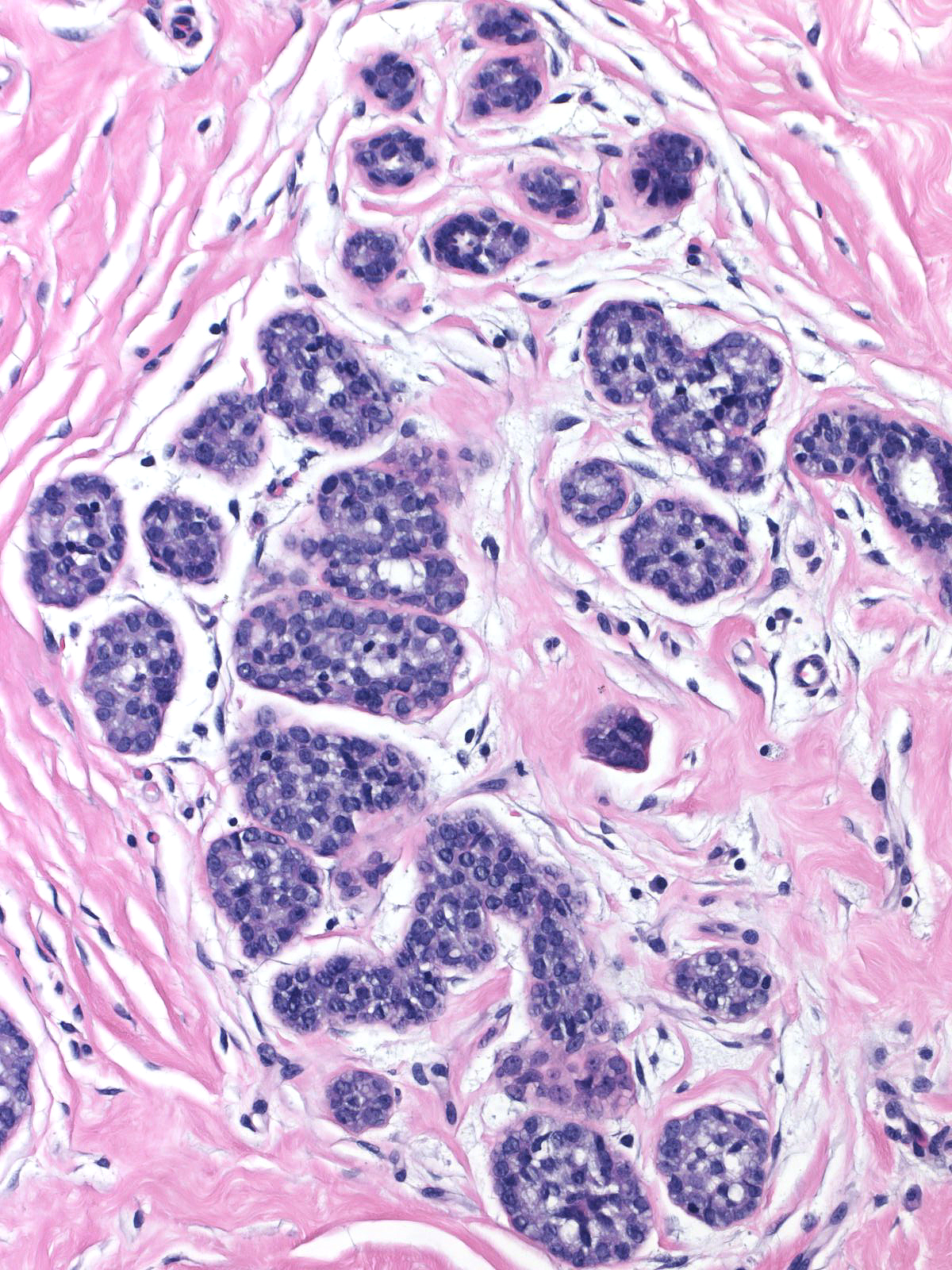
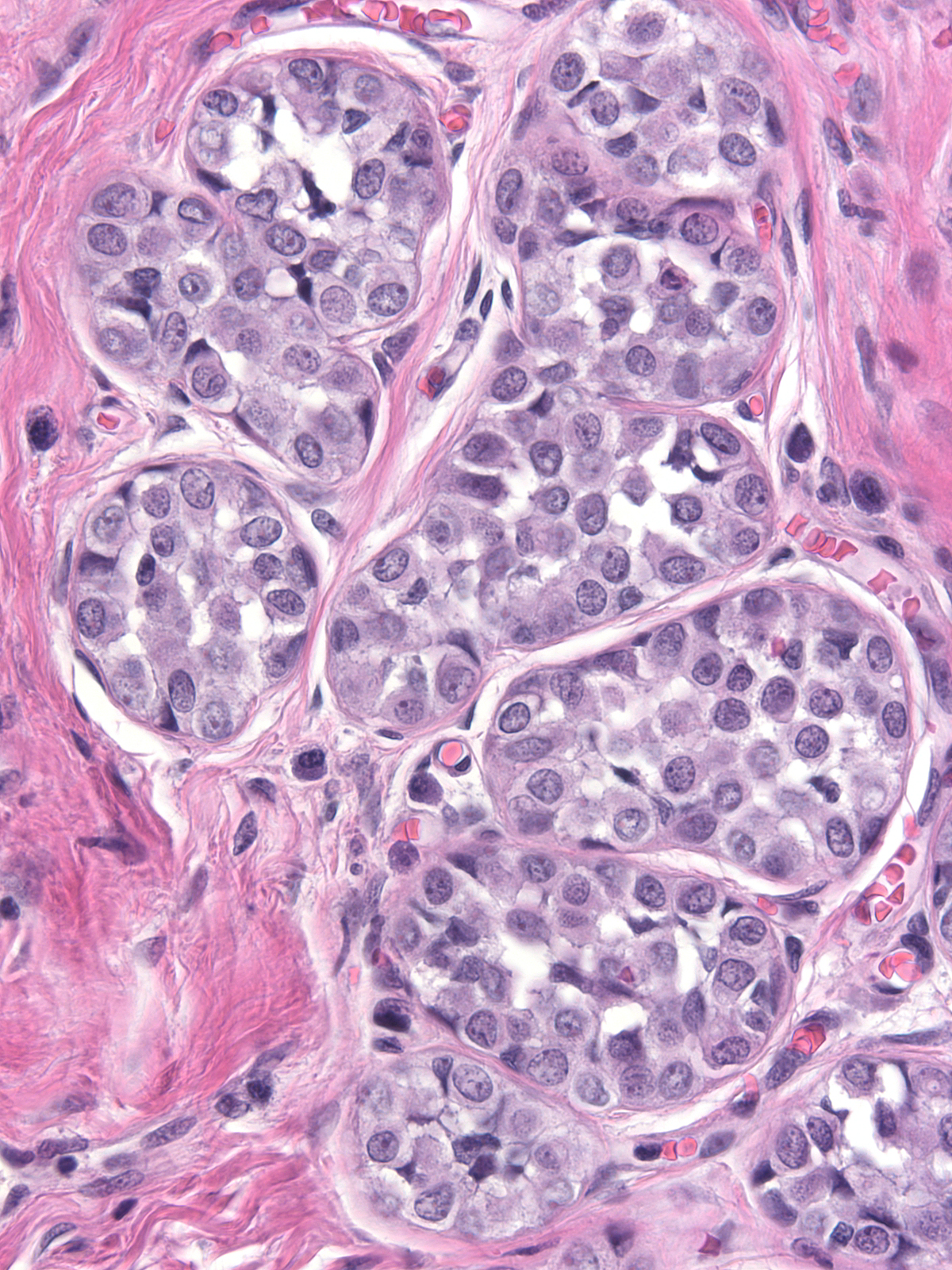
Pathologists have proposed the use of several parameters to estimate the number of the neoplastic cells: number of lobules involved, extent of involvement within a lobule, and degree of acinar distension or distortion. Contemporary thinking allows the diagnosis of lobular carcinoma in-situ for foci in which the neoplastic cells completely fill, distort, and distend at least 50% of the acini in a single lobule (certain authors suggest a higher threshold of 75%). Lesser involvement of a single lobule merits the diagnosis of atypical lobular hyperplasia. Here are a few examples of atypical lobular hyperplasia:
| In this example of ALH, the neoplastic lobular cells involve almost every acinus of the lobule but most acini do not appear distorted or greatly distended when compared to uninvolved acini. | 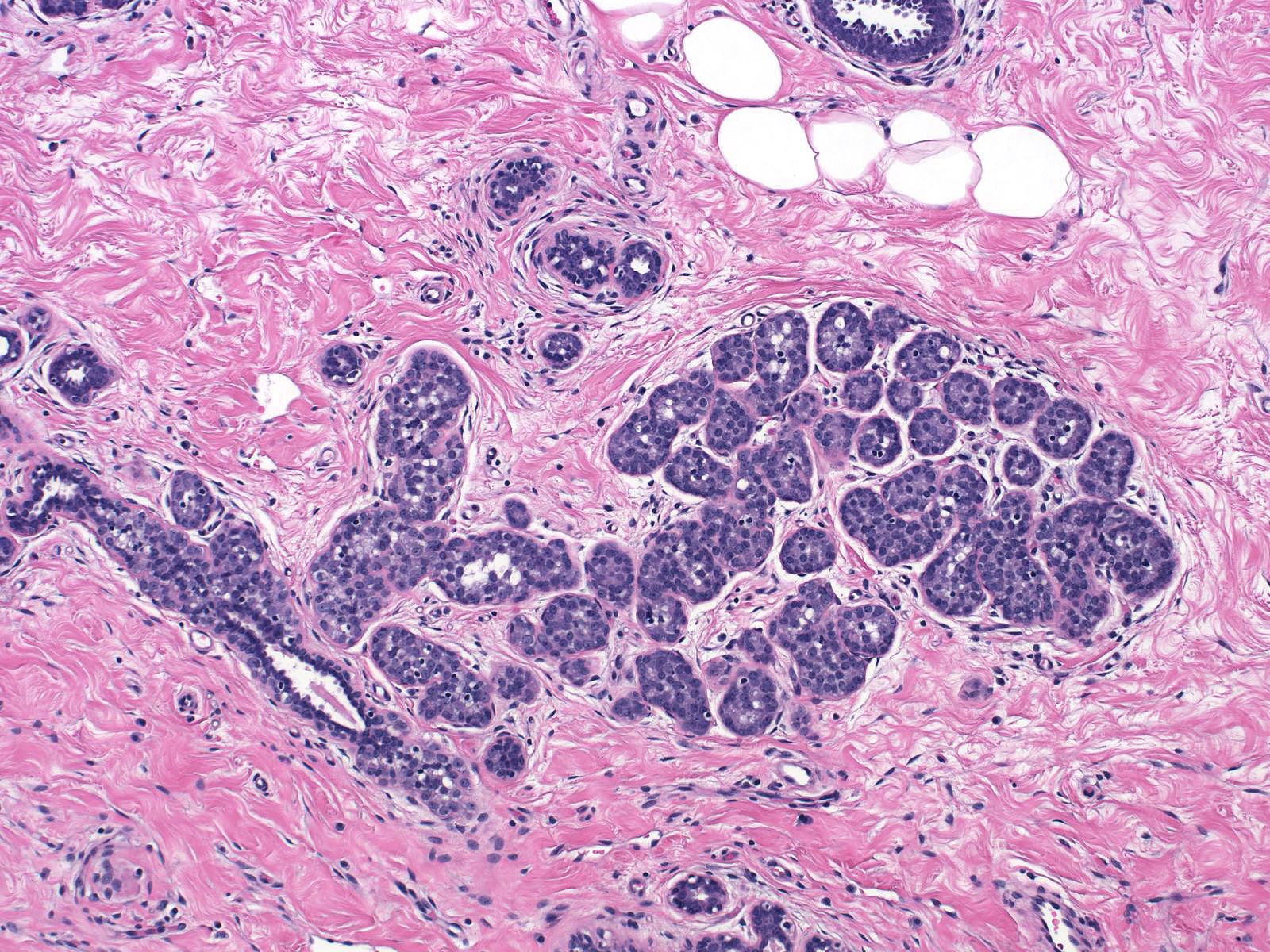 |
| In this example of ALH, the acini and ductules do not appear significantly distended or distorted by the neoplastic population. | 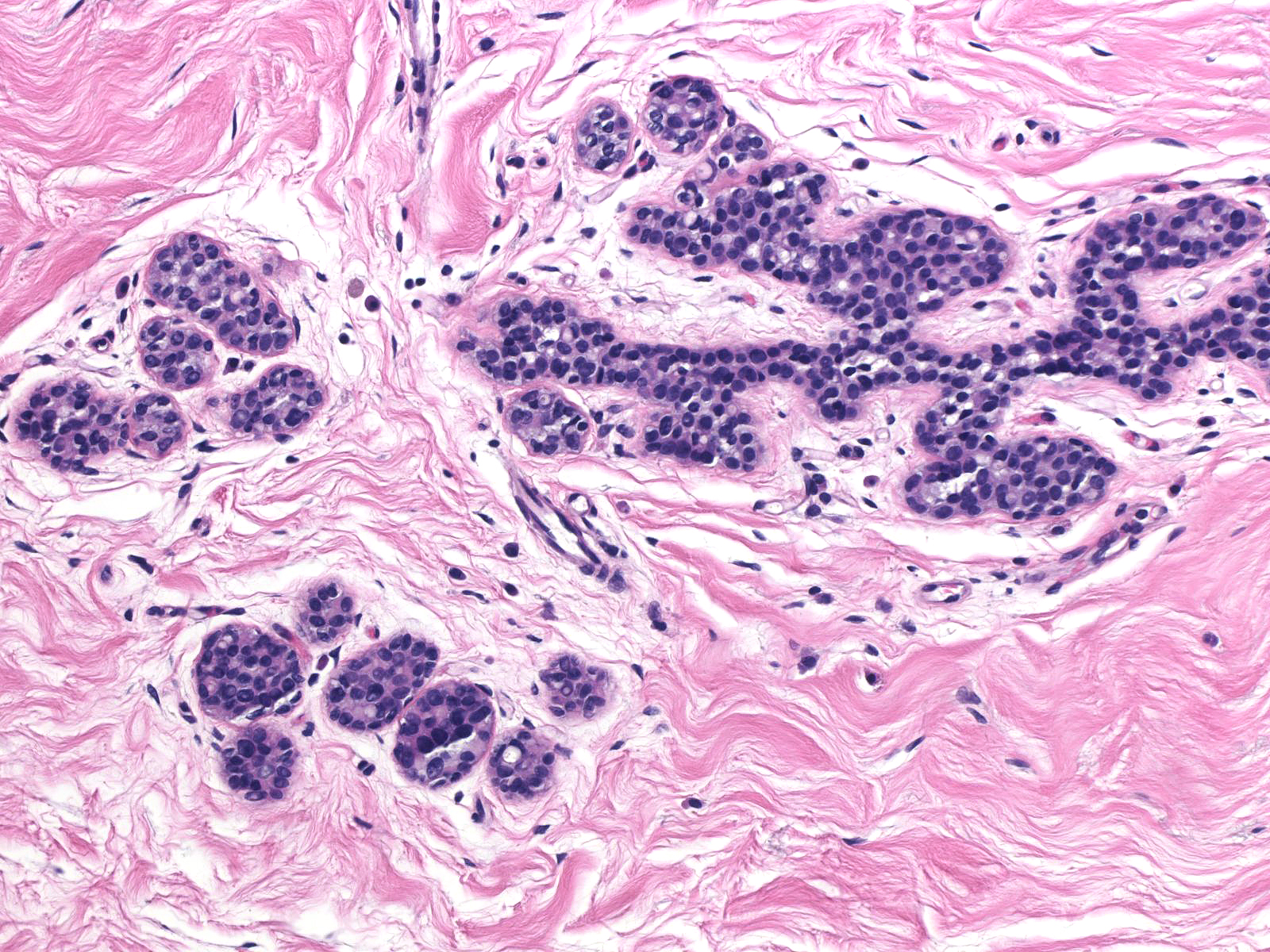 |
| Preservation of lumens in many of the acini in this lobule indicates that the neoplastic population does not completely fill and distend the acini. Atypical, dishesive cells comprise perhaps 25% of the cellular population and a diagnosis of ALH is appropriate. | 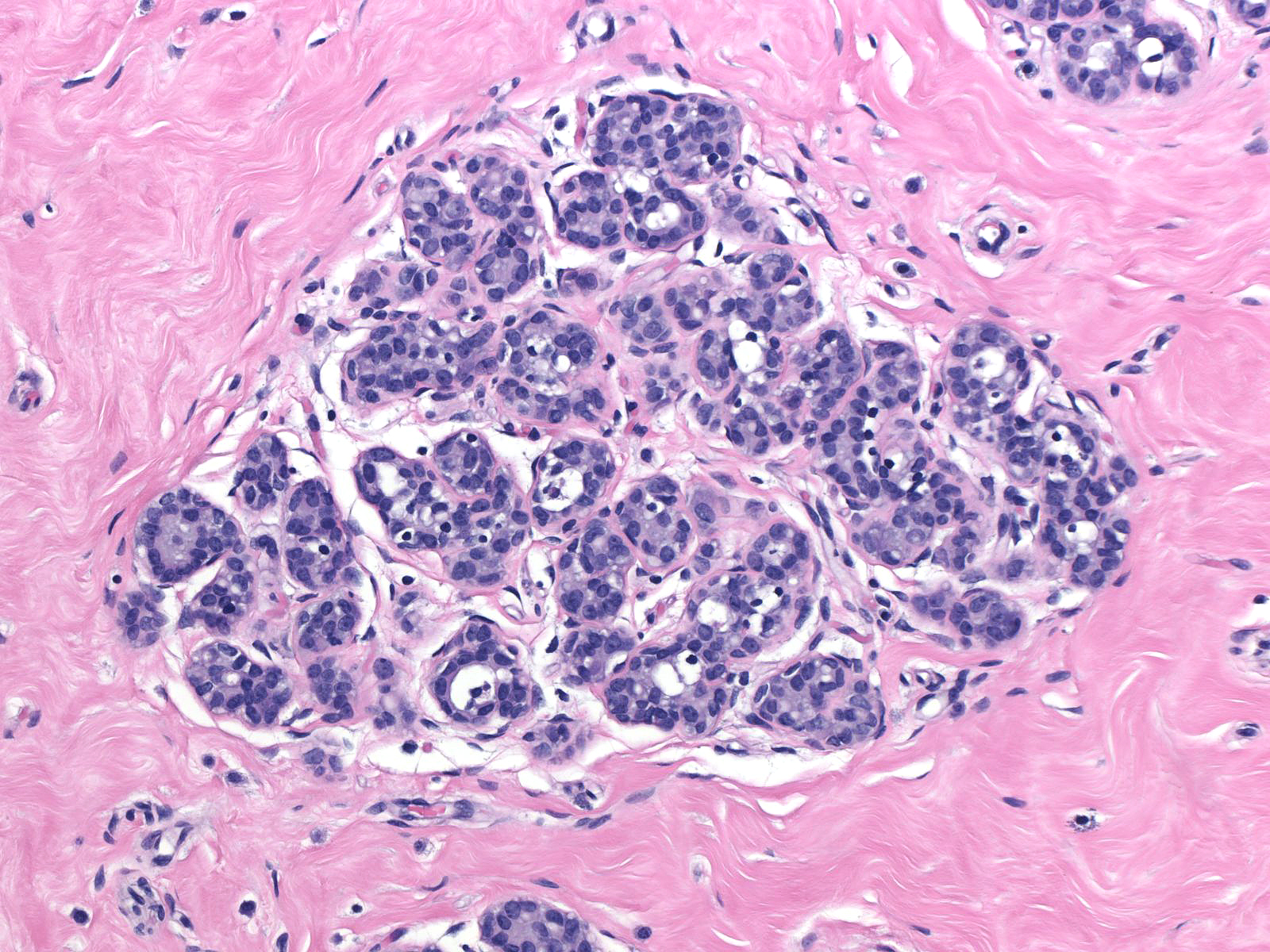 |
Comparison of the lobule involved by lobular neoplasia (left image) to a nearby uninvolved lobule (right image) reveals only minimal distention of the acini without distortion and with preservation of many lumens.
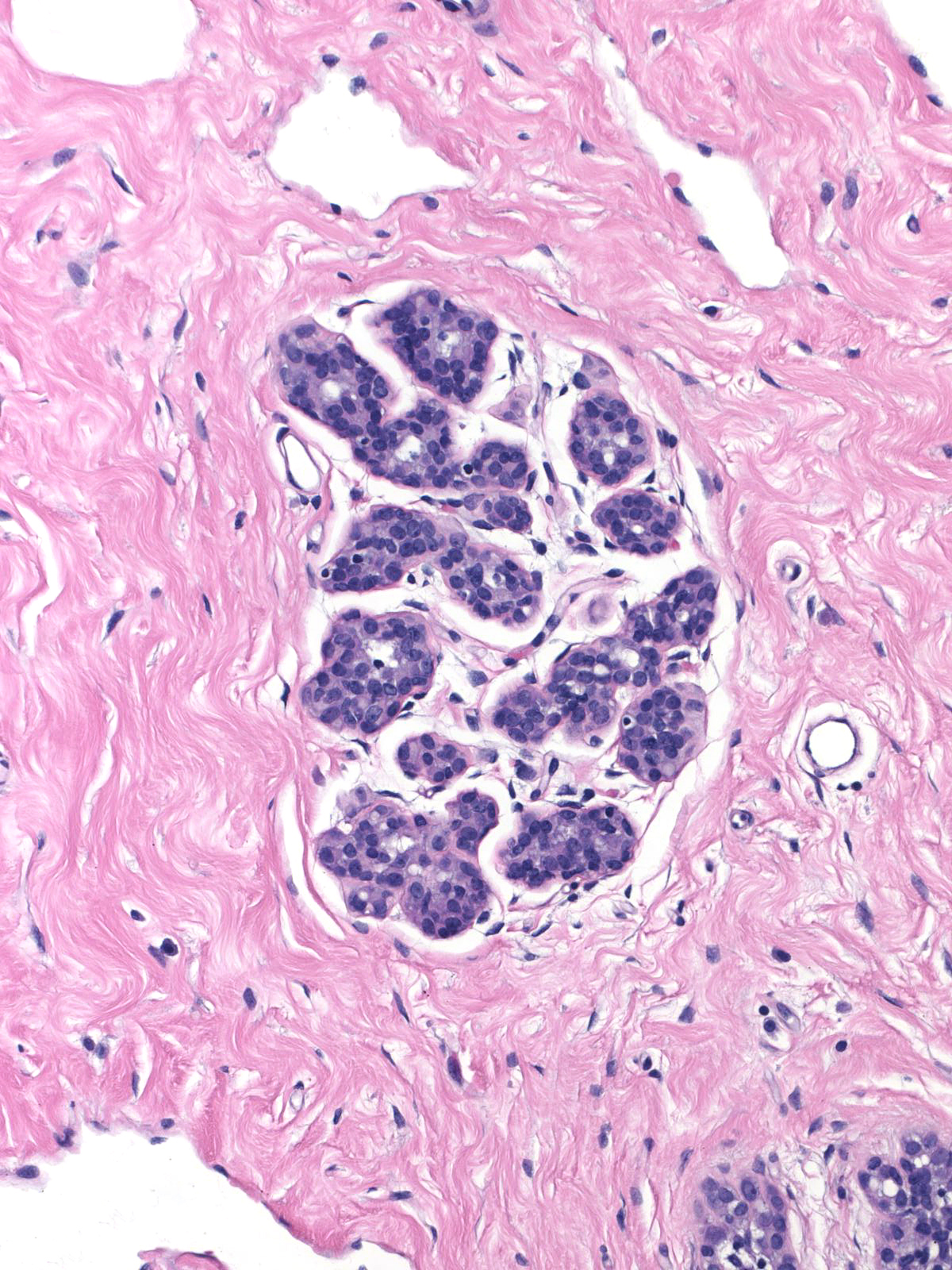 |
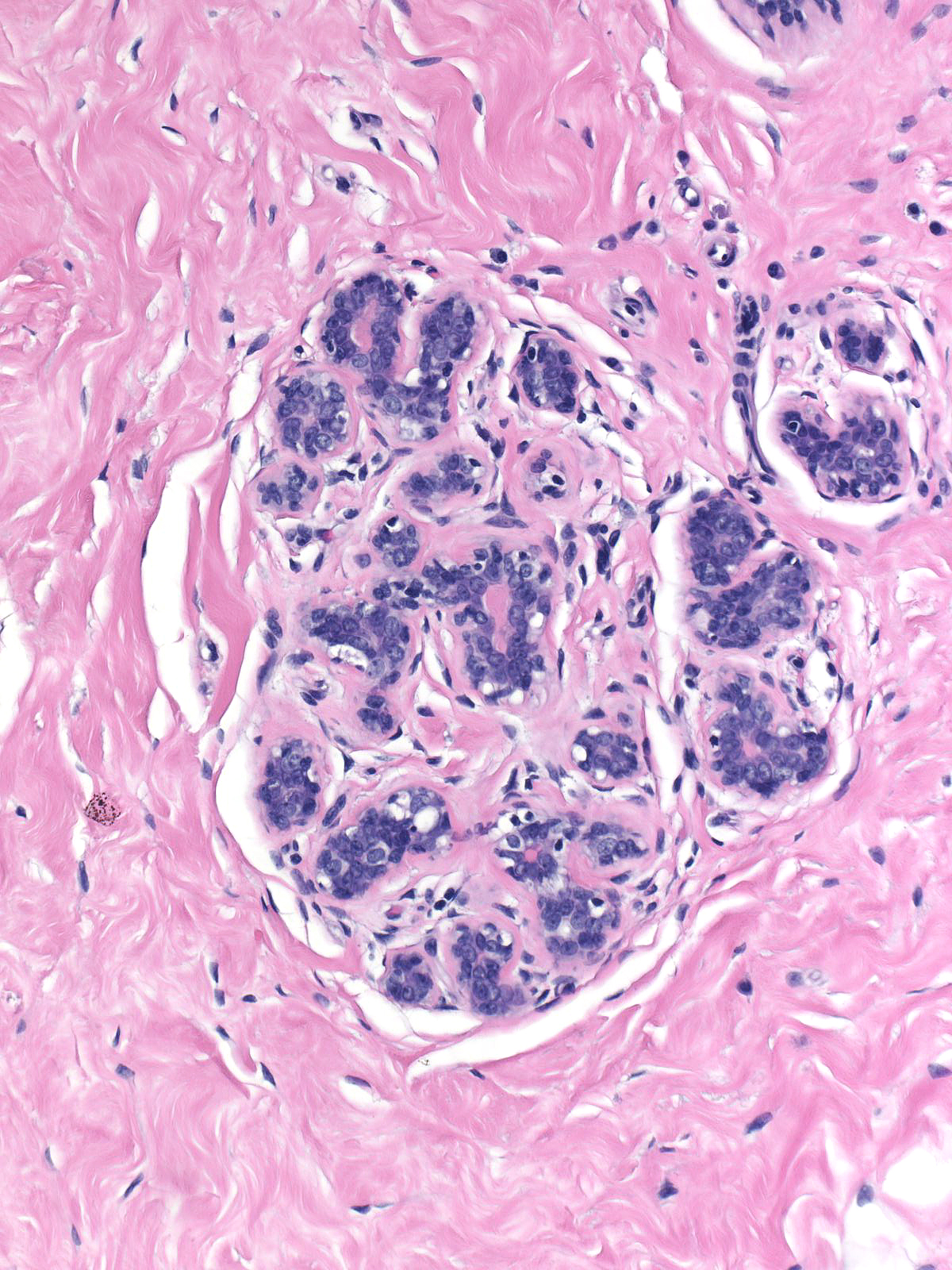 |
Although one can evaluate the criteria for lobular neoplasia in unaltered breast tissue, it becomes impossible to assess them in the presence of co-existing glandular alterations. For example, postmenopausal atrophy leads to shrinkage of acini, which complicates the estimation of acinar distention. Similarly, irradiation alters the structure and cellular composition of acini.
 |
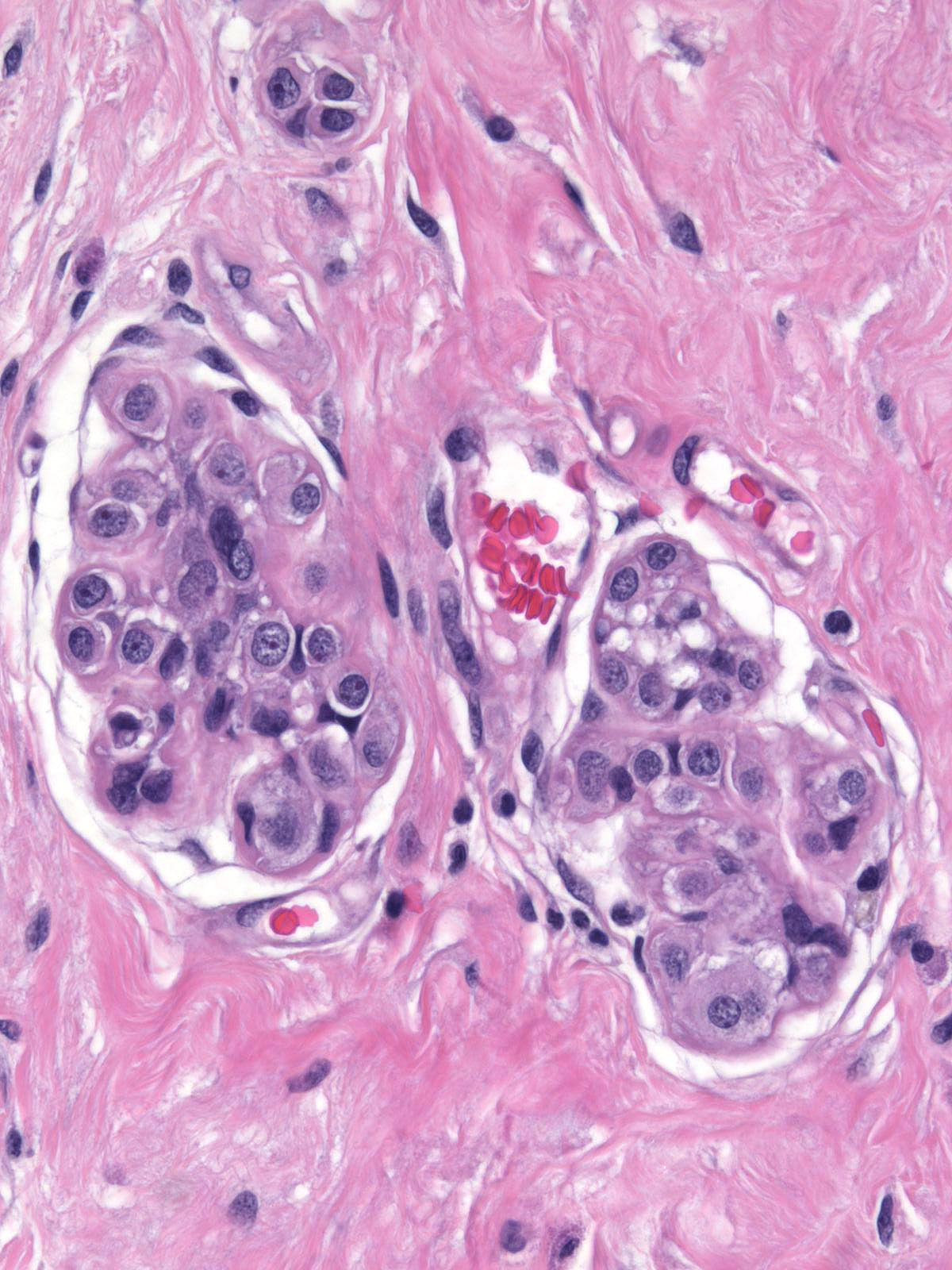 |
Structural distortion of lobules by processes such as sclerosing adenosis and collagenous spherulosis make it impossible to judge both the acinar size and the extent of involvement.
 |
 |
The glandular tissue in papillomas and fibroadenomas does not consist of genuine lobules, making it impossible to impossible to evaluate the extent of the lobular involvement.
 |
 |
Furthermore, one cannot rely on criteria appropriate for lobules when evaluating lobular neoplasia involving ducts.
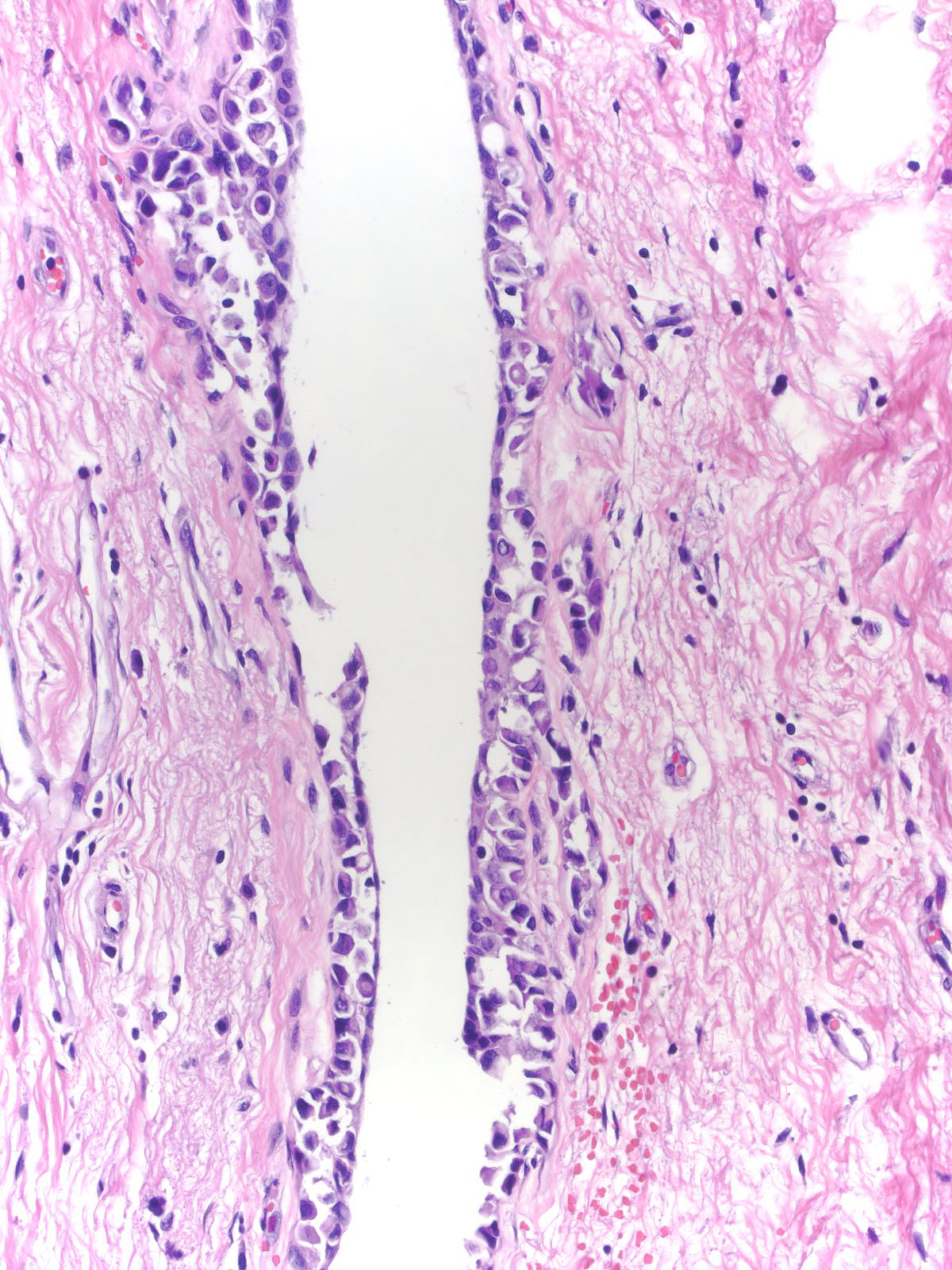 |
 |
Keep in mind that the proposed criteria for separating atypical lobular hyperplasia and lobular carcinoma in-situ represent nothing more than a not yet validated convention based on popular agreement. Clinical follow up studies have not consistently demonstrated differences in the risks for the development of invasive carcinoma associated with atypical lobular hyperplasia and lobular carcinoma in-situ. The borderline between atypical lobular hyperplasia and lobular carcinoma in-situ remains undefined and the distinction remains subjective.
Differential Diagnosis
The distinction of lobular neoplasia from pagetoid growth of ductal carcinoma cells, prominent clear basal cells, and immature ductal hyperplasia represents a more relevant problem.
When ductal carcinoma in-situ grows as scattered cells dispersed among pre-existing benign epithelial cells, it can mimic the appearance of atypical lobular hyperplasia (left). Almost never does ductal carcinoma grow solely in a pagetoid fashion; consequently, when trying to differentiate pagetoid DCIS from ALH, you should look carefully for co-existing foci of ductal carcinoma in-situ exhibiting a conventional growth pattern (right). Evidence of cellular polarization and cohesion and the presence of E-cadherin will also help to distinguish pagetoid DCIS from atypical lobular hyperplasia.
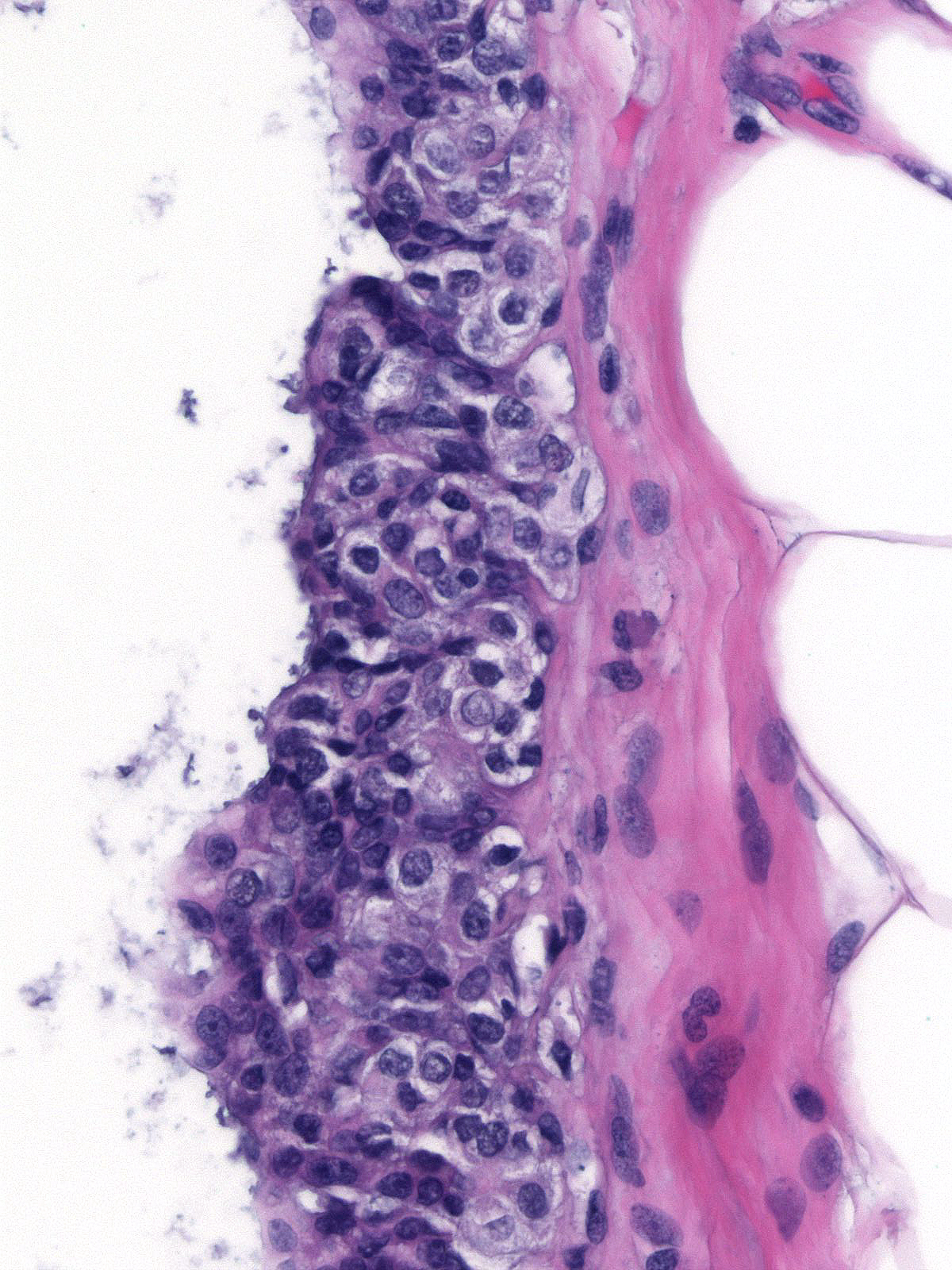 |
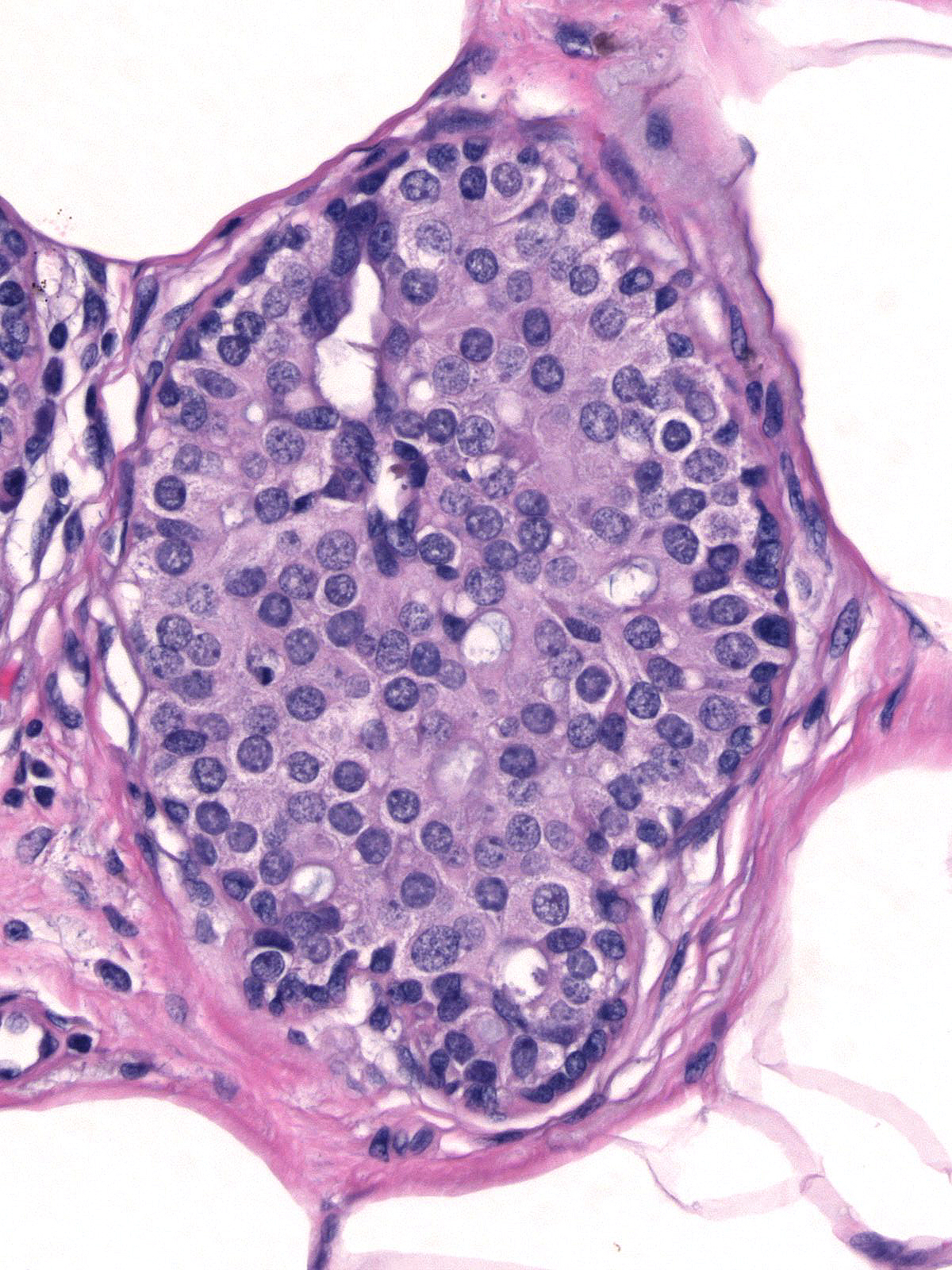 |
| At times, basal cells develop clear cytoplasm and thereby attract attention. | 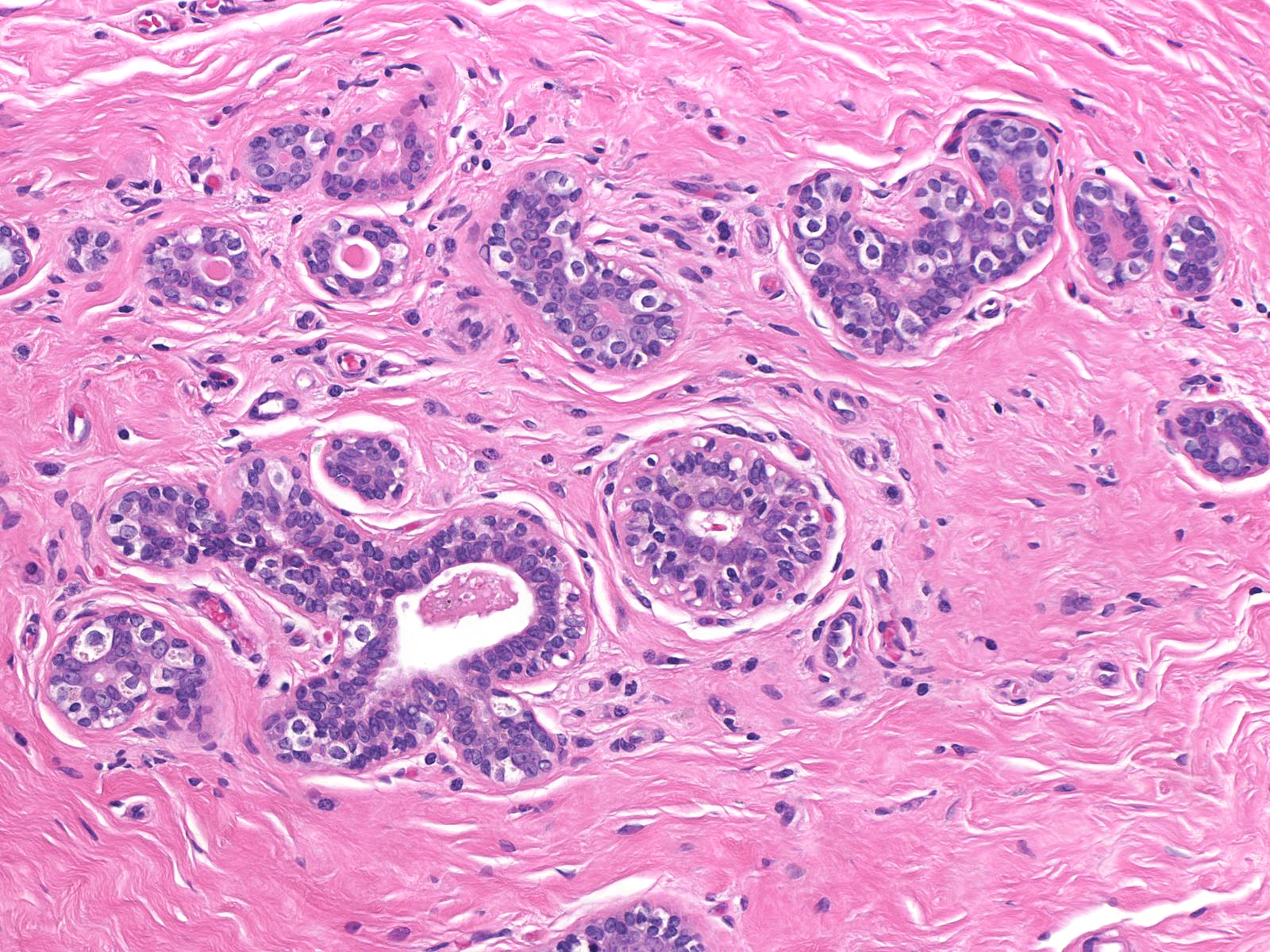 |
When contrasted with neoplastic lobular cells, clear basal cells have abundant pale cytoplasm, which occasionally contains miniscule, eosinophilic aggregates of cytoplasm. It does not display the finely vacuolated, foamy or frothy quality seen in neoplastic lobular cells. Moreover, clear basal cell cells do not usually form clusters.
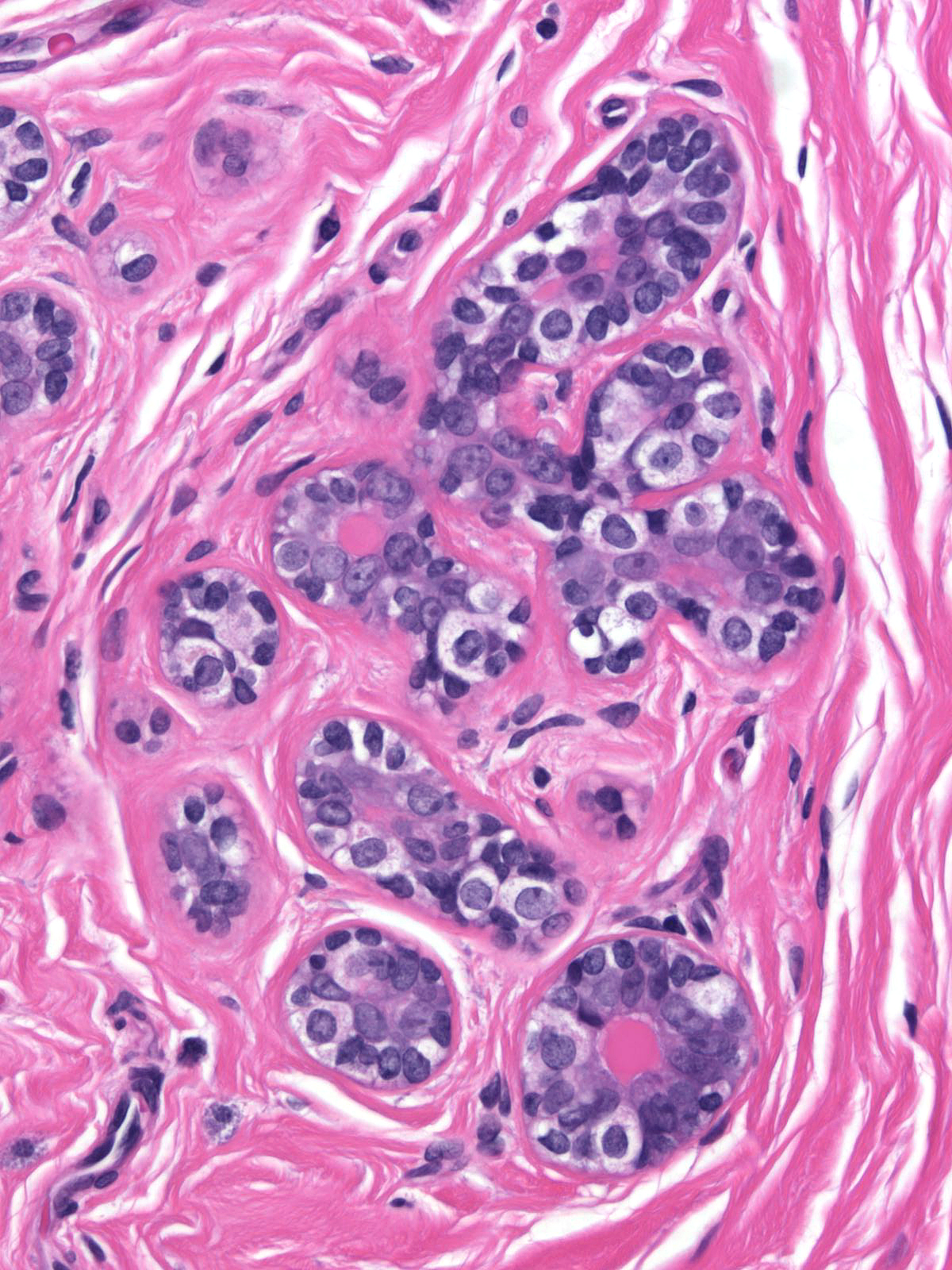 |
 |
| Immature hyperplastic ductal cells can also resemble those of lobular neoplasia. | 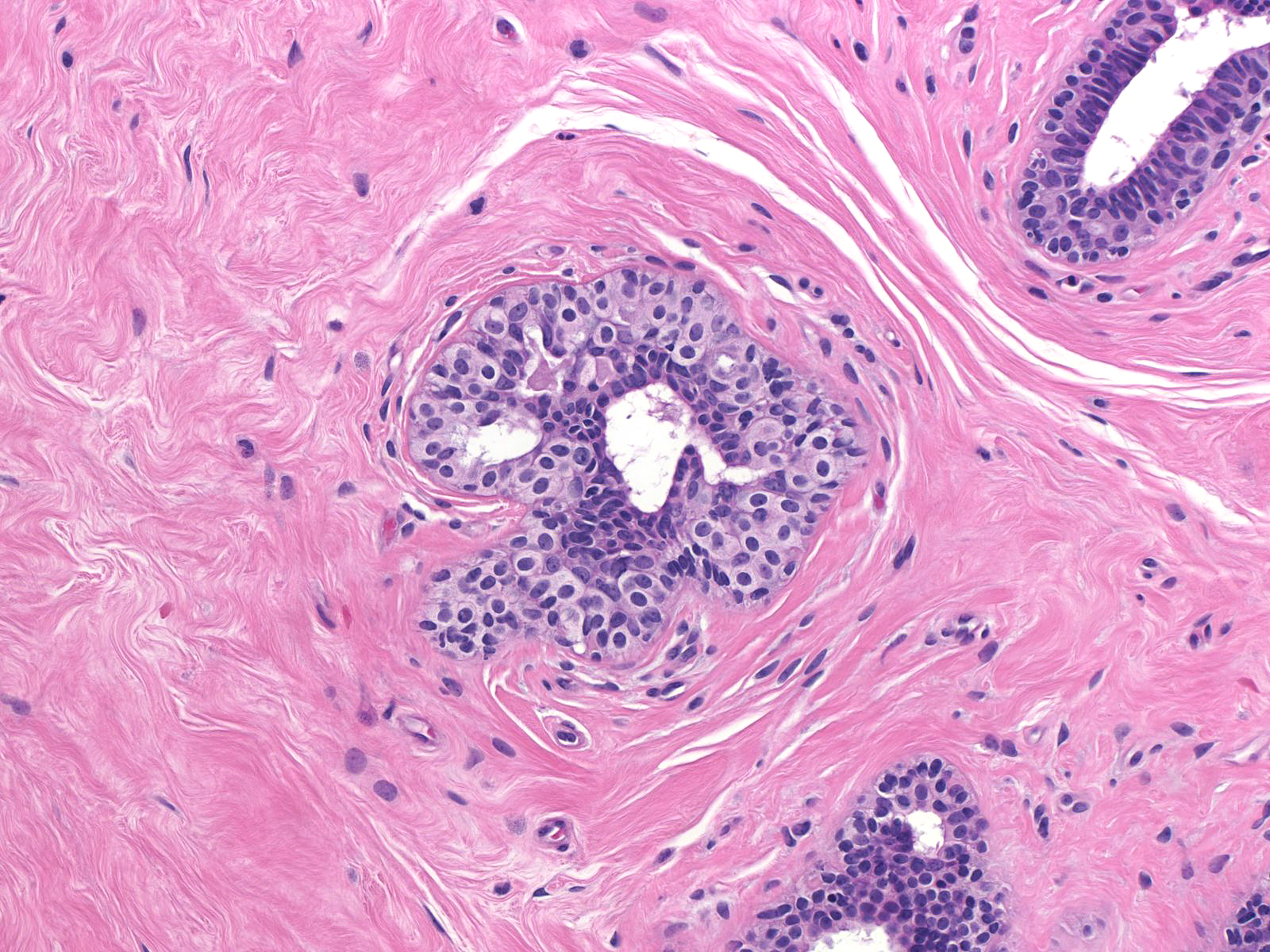 |
Immature hyperplastic ductal cells possess moderate amounts of finely granular cytoplasm, which lacks microvesicles. The nuclei usually have smoothly contoured oval shapes and homogeneous chromatin. These features contrast those of neoplastic lobular cells, which contain small amounts of frothy cytoplasm and nuclei showing slightly variable contours and containing finely granular chromatin. Moreover, immature hyperplastic ductal cells typically merge with hyperplastic ductal cells showing the usual morphological attributes.
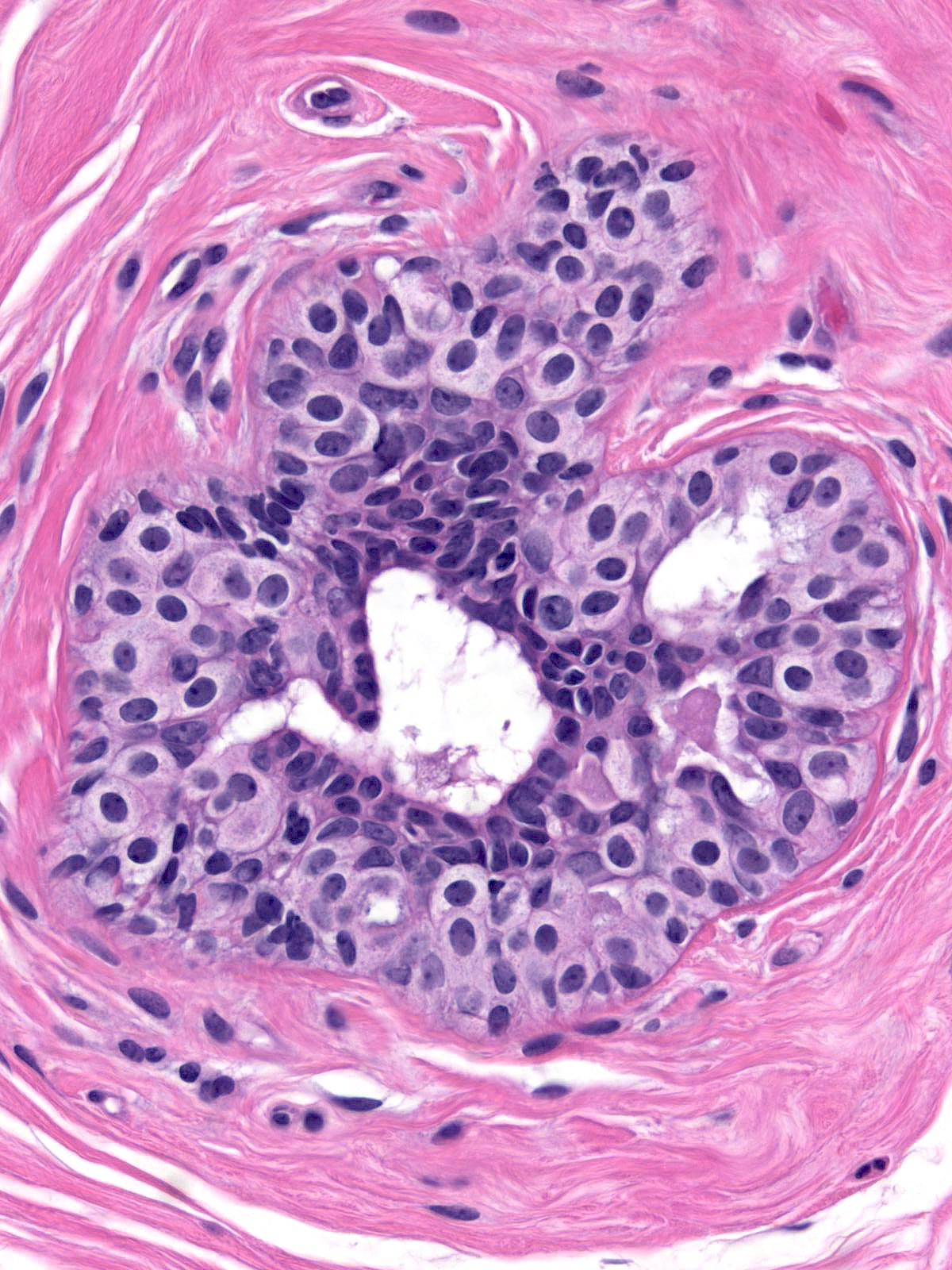 |
 |
| Like the cells of lobular carcinoma in-situ, those of atypical lobular hyperplasia do not stain for E-cadherin, a feature that can aid in the diagnosis. Beware of antigenicity loss in poorly-fixed tissue which can cause spurious E-cadherin negativity. | 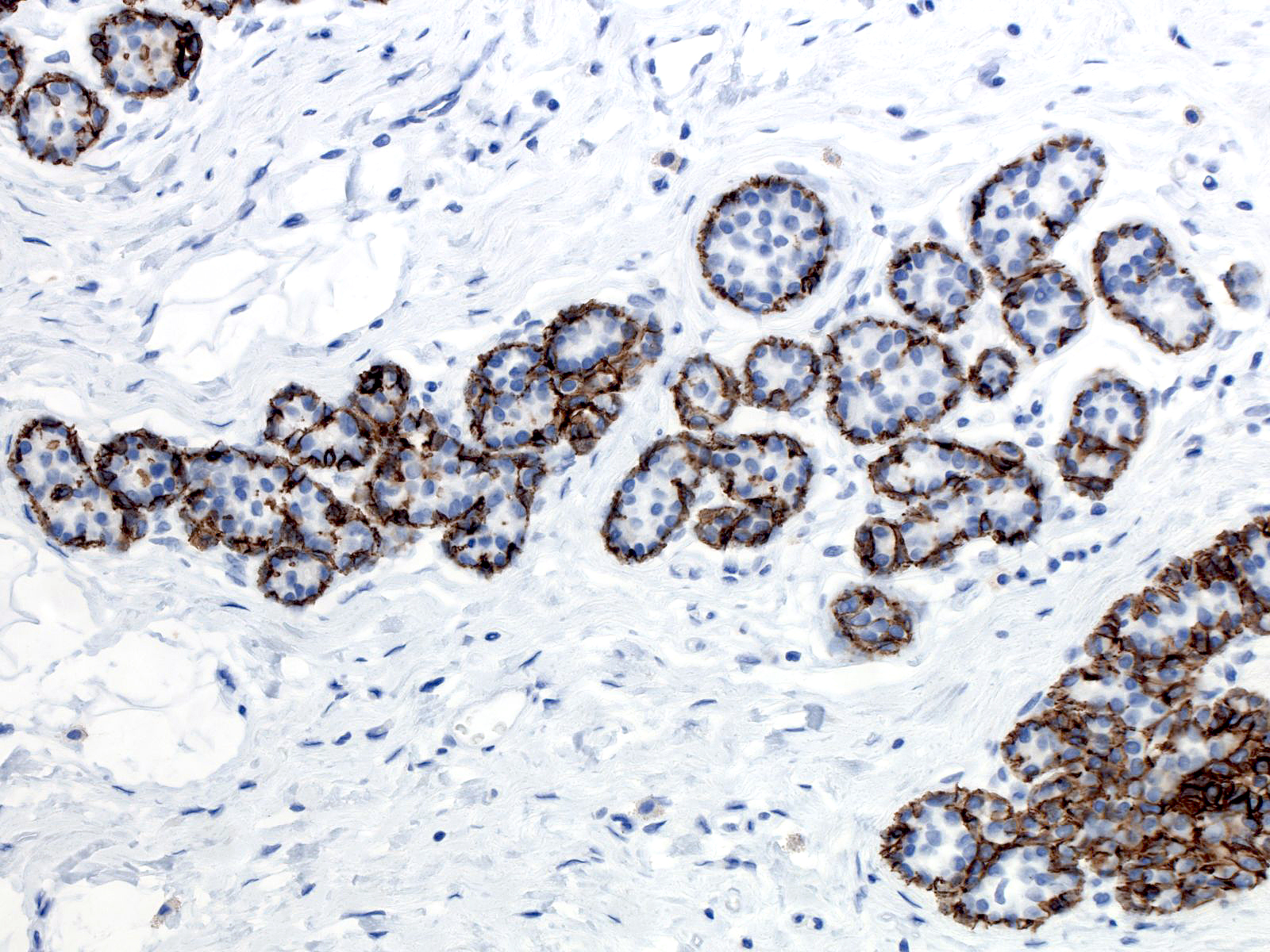 |
tumor) can demonstrate this finding.|24-7 Beta_cat.jpg}}