Difference between revisions of "Atypical Ductal Hyperplasia (ADH)"
| Line 32: | Line 32: | ||
{{img3|20-16 IH.jpg|20-17 Peri.jpg|20-18 RADS-2.jpg|Immature hyperplasia|Hyperplasia in perimenopause|Hyperplasia within a radial scar}} | {{img3|20-16 IH.jpg|20-17 Peri.jpg|20-18 RADS-2.jpg|Immature hyperplasia|Hyperplasia in perimenopause|Hyperplasia within a radial scar}} | ||
tumor) can demonstrate this finding.|24-7 Beta_cat.jpg}} | tumor) can demonstrate this finding.|24-7 Beta_cat.jpg}} | ||
| − | + | {{:mgh:breast-footer}} | |
| − | |||
| − | |||
Revision as of 18:12, July 13, 2020
Contents
General
- Definition: Atypical ductal hyperplasia (ADH) is a proliferation of atypical ductal cells demonstrating low-grade cytological atypia but lacking well-developed architectural atypia. Extremely small foci showing morphological features of low‑grade ductal carcinoma in-situ are also usually classified as atypical ductal hyperplasia.
- Clinical Significance: Atypical ductal hyperplasia can give rise to calcifications, but it does not ordinarily produce clinical signs or symptoms. Oncologists usually recommend antiestrogen therapy for patients with atypical ductal hyperplasia to reduce the likelihood of the development of carcinoma. When a core biopsy reveals atypical ductal hyperplasia, a surgeon will usually carry out an excision of the targeted area to exclude the presence of carcinoma.
- Gross Findings: None
- Microscopic Findings: Low-grade cytological atypia and cellular stratification that lacks clear-cut architectural atypia characterize ADH. Occasional examples display the cytological and architectural features of low-grade ductal carcinoma in-situ but lack the extent of proliferation needed to establish the diagnosis of carcinoma in-situ.
- Differential Diagnosis: Usual ductal hyperplasia (nonpolarized cells, no cytological or architectural atypia), flat epithelial atypia (no stratification), ductal carcinoma in-situ (well developed architectural atypia, robust proliferation)
- Discussion: The morphological patterns created during the formation of ductal carcinoma in-situ form a continuum that stretches from flat epithelial atypia to low‑grade ductal carcinoma in-situ. Atypical ductal hyperplasia occupies a position in the middle of this continuum.
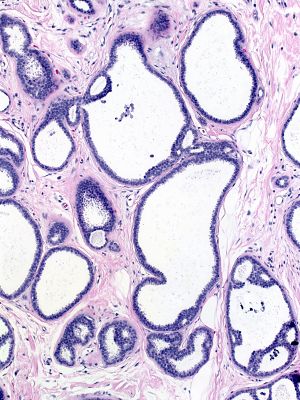
| This image illustrates the patterns seen in this neoplastic spectrum. FEA appears in the bottom left corner and DCIS in the right of the image. The proliferation in a few central glands merits the diagnosis of atypical ductal hyperplasia. |  |
The lesions that make up this continuum display similar cytological features. The presence of low‑grade atypia represents the most important of these features, for it stands as a defining characteristic of the low‑grade continuum. One cannot make a diagnosis of any of the lesions in this pathway in its absence. The composite image below illustrates the similarity of the nuclear features throughout this continuum.
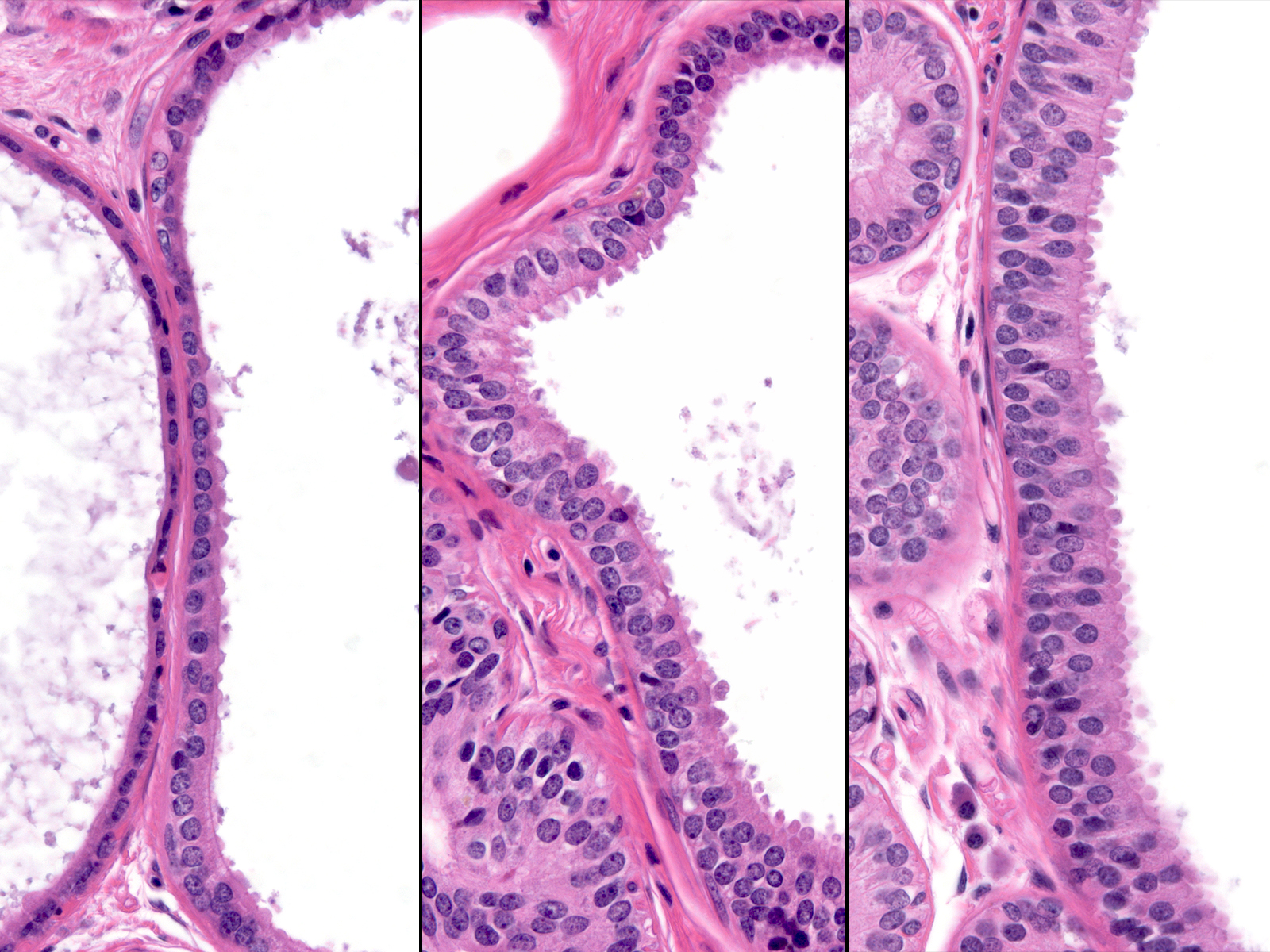 |
 |
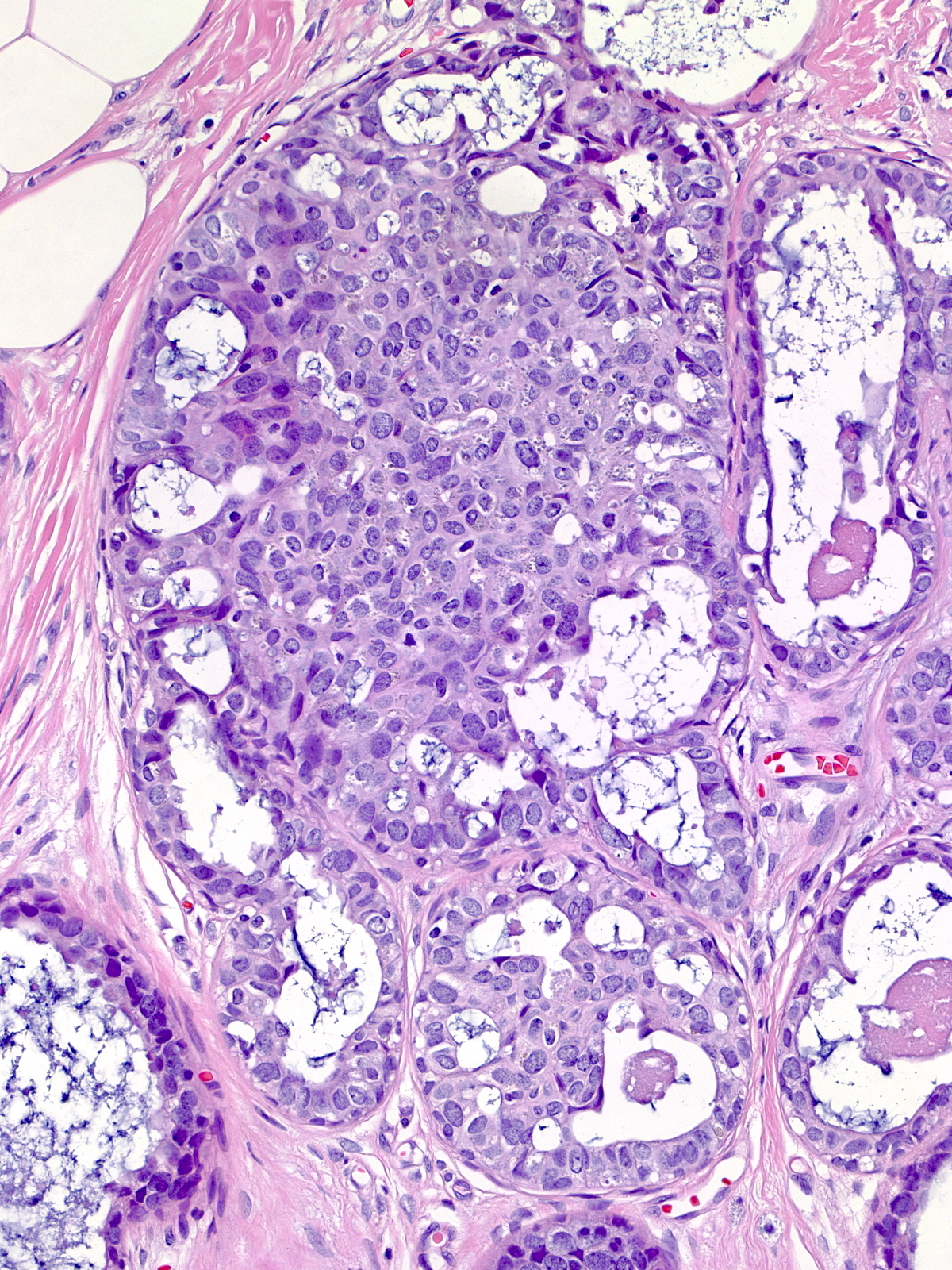
Because the cytological properties of the lesions in this continuum appear similar, one uses architectural characteristics to divide this continuum into three stages. When the neoplastic cells grow in a flat pattern, they represent flat epithelial atypia, whereas stratification indicates a more advanced form of ductal atypia (either atypical ductal hyperplasia or ductal carcinoma in-situ). Examples showing modest degrees of cellular proliferation and lacking well-developed architectural atypia merit the diagnosis of atypical ductal hyperplasia.
 |
 |
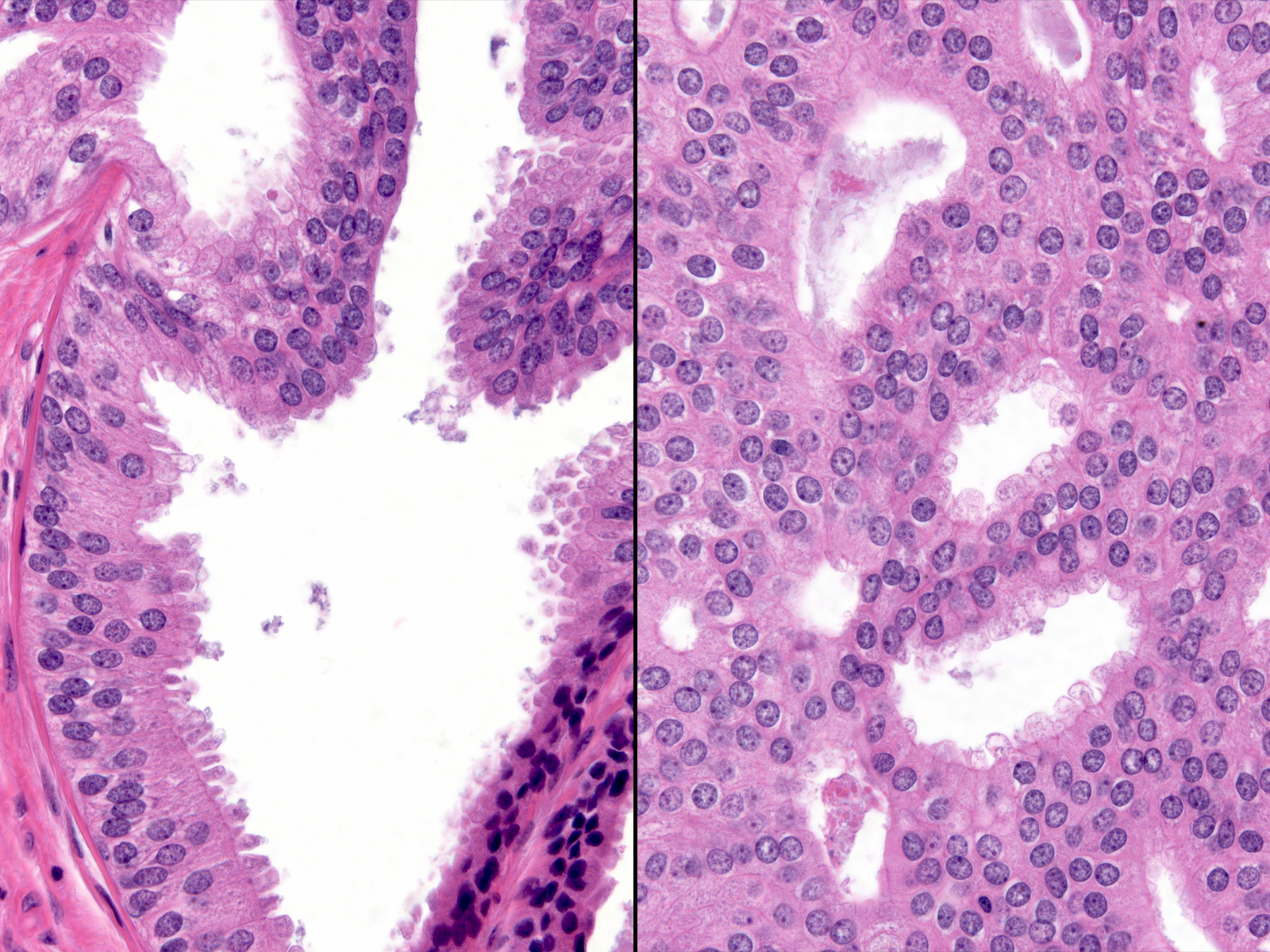
| When the neoplastic cells demonstrate stratification and clear-cut architectural atypia and they distend the involved glands noticeably, one can make the diagnosis of low-grade ductal carcinoma in-situ. |  |
Pathologists usually also require the presence of these changes in several glands or spanning 0.2 cm to establish the diagnosis of ductal carcinoma in-situ. Occasional cases provoke controversy in their classification, usually because they consist of very small foci or because the evidence of architectural atypia seems limited or poorly developed.
 |
 |
 |
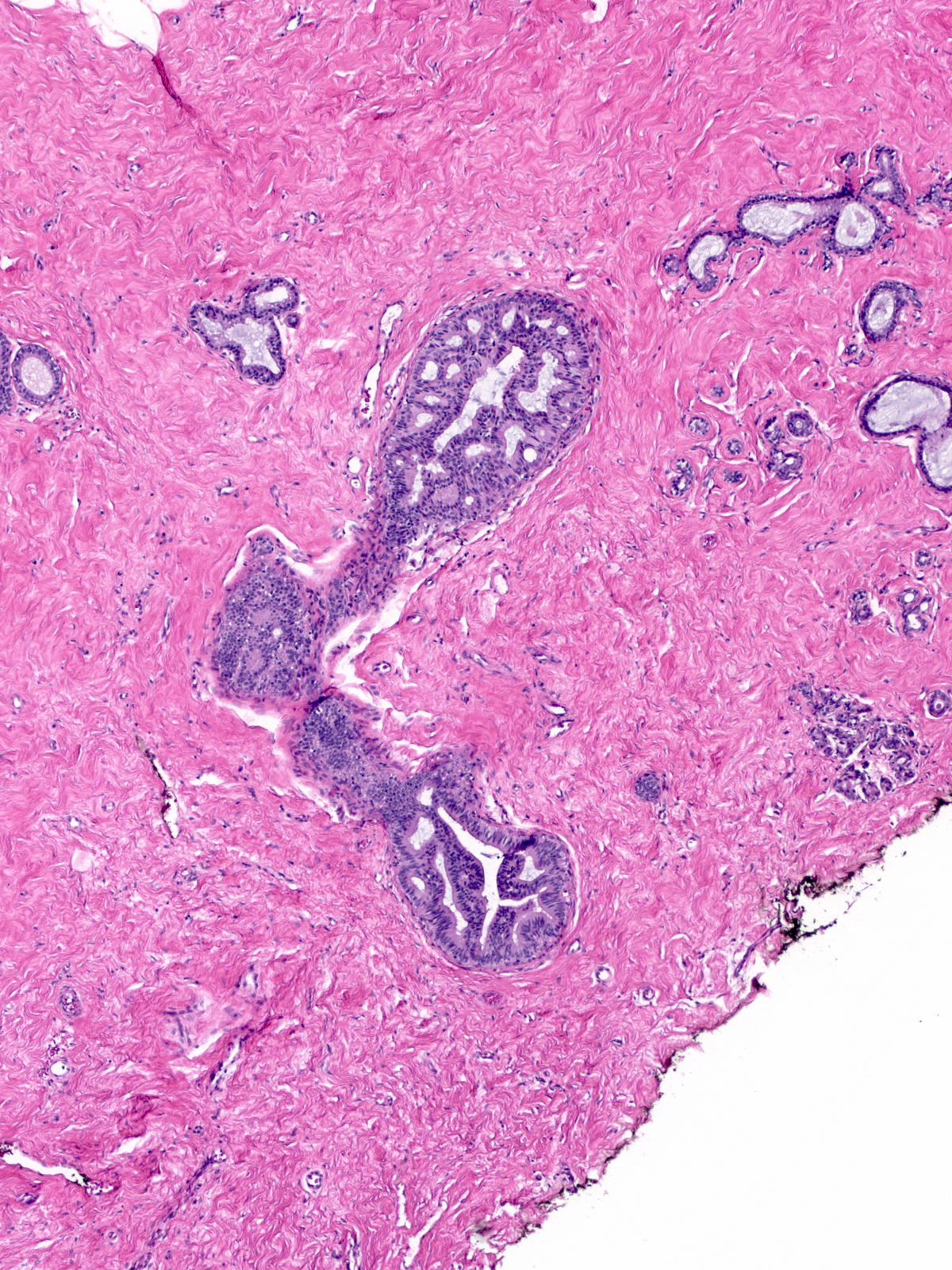
Pathologists will disagree about the diagnosis of such proliferations and often classify them as borderline. One must take care to limit the diagnosis of ADH to foci displaying true cytologic atypia and proliferation, as artifacts of tissue sectioning or specimen handling commonly create mimics. For example, off-center planes of section can create the impression of cellular stratification when none exists. In these images we see the same duct in two sections one level apart. The image on the left represents a tangential cut through the wall of the duct rather than a cellular proliferation.
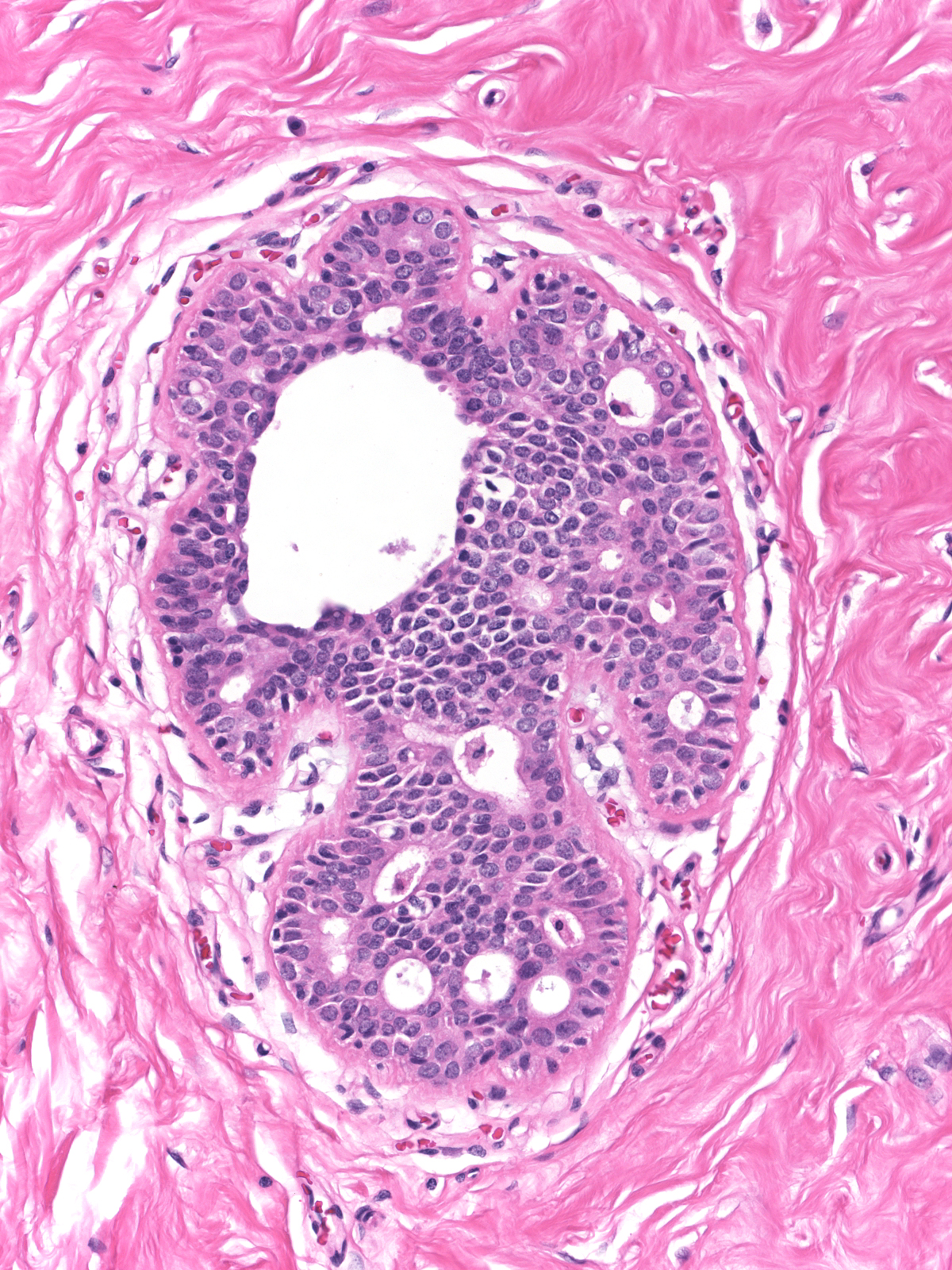 |
 |
| Cauterization of usual ductal hyperplasia can also look very atypical. Interpret regions of cautery artifact and poor preservation with caution. (UDH with cautery artifact) |  |
| Several forms of usual ductal hyperplasia can appear somewhat unusual. Florid hyperplasia, as seen in this image, does not merit the diagnosis of atypical ductal hyperplasia. | 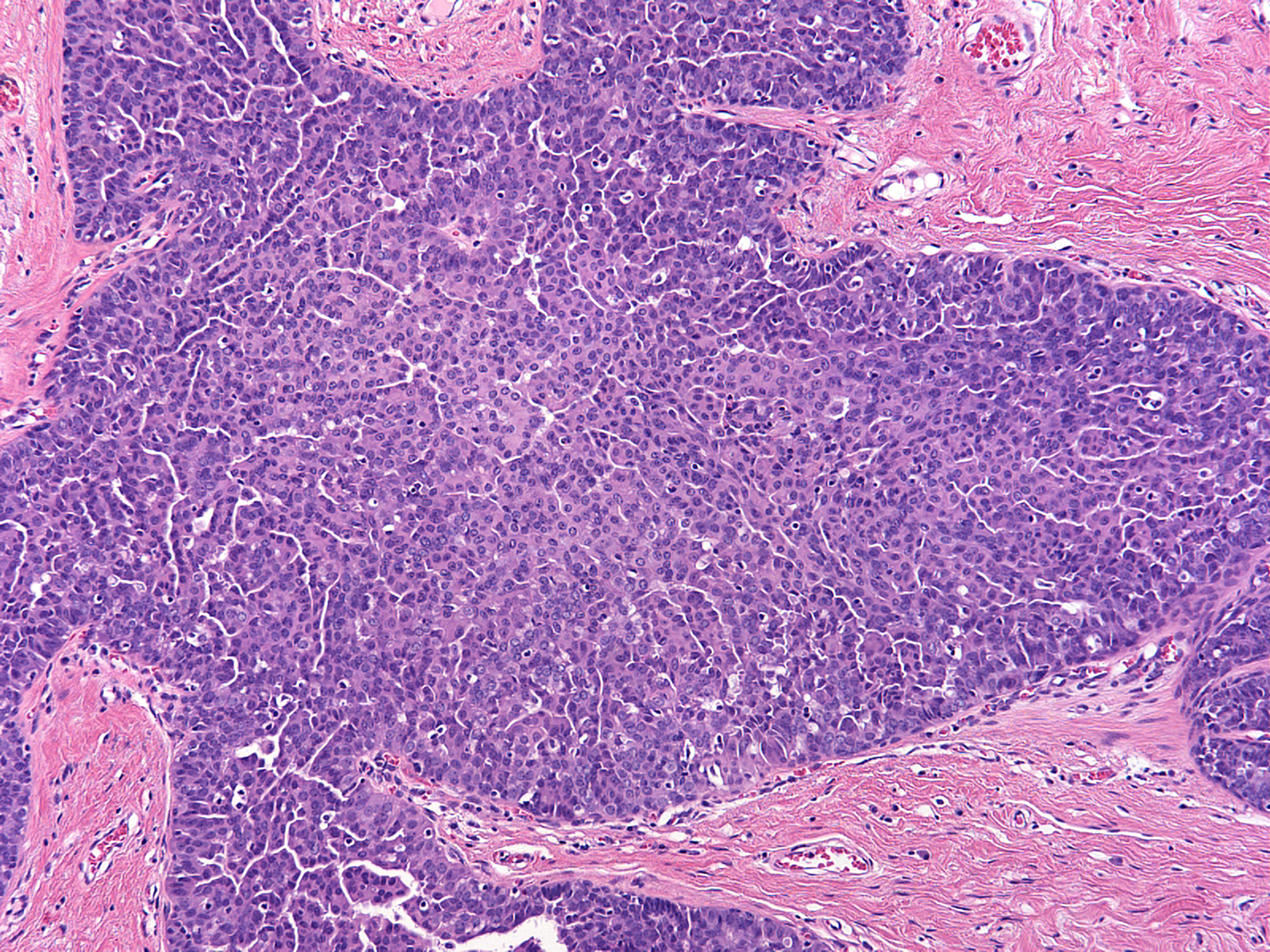 |
| In other uncommon examples, the nuclei of usual ductal hyperplasia contain finely dispersed dark chromatin. This finding by itself does not indicate the presence of atypia. (UDH with dark nuclei) | 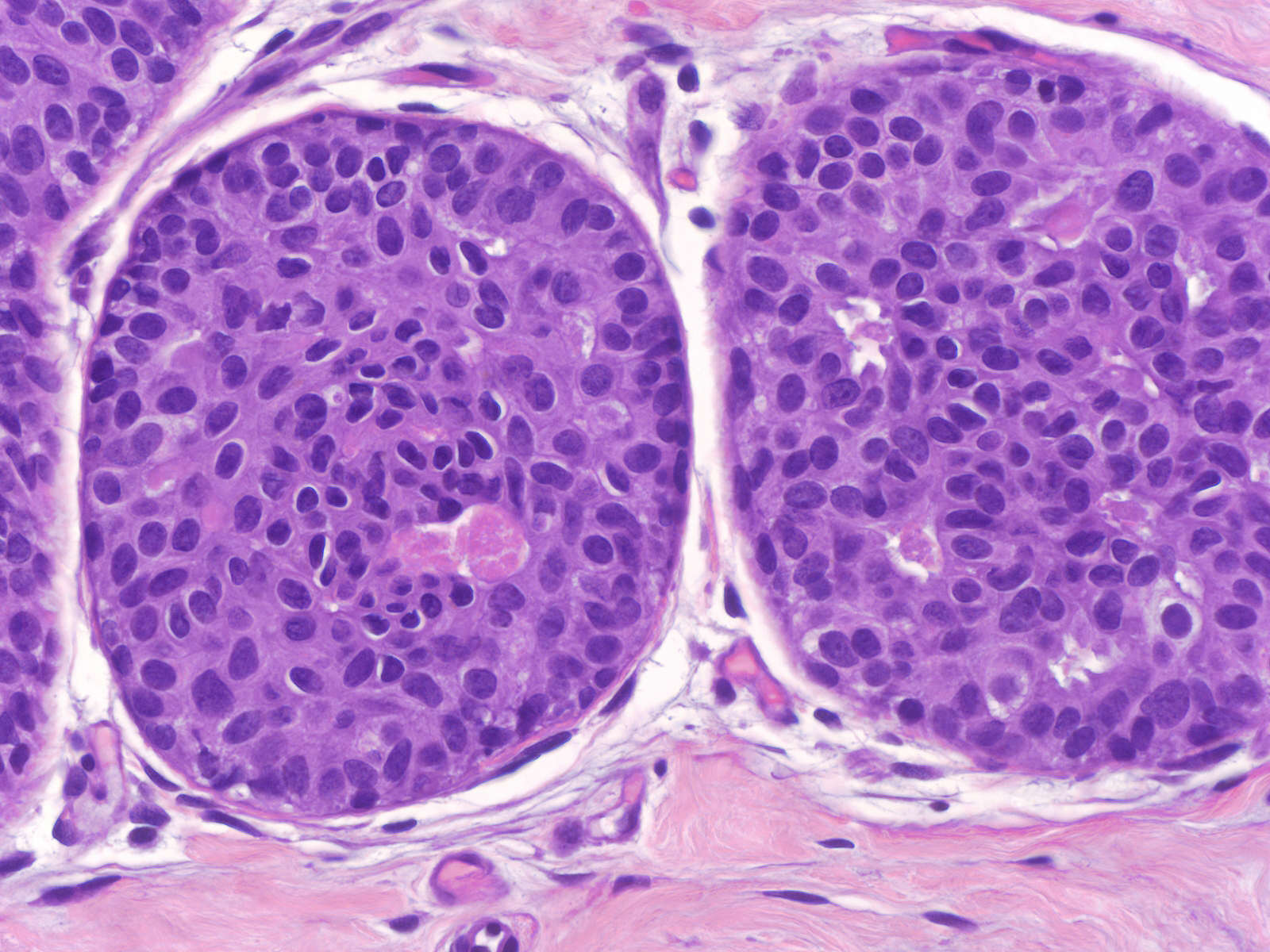 |
Immature hyperplasia, hyperplasia in perimenopausal women, and hyperplasia within the coronas of radial scars can all display cytological features that suggest atypia. Only after especially careful study and consideration should one should make the diagnosis of atypical ductal hyperplasia in these situations.
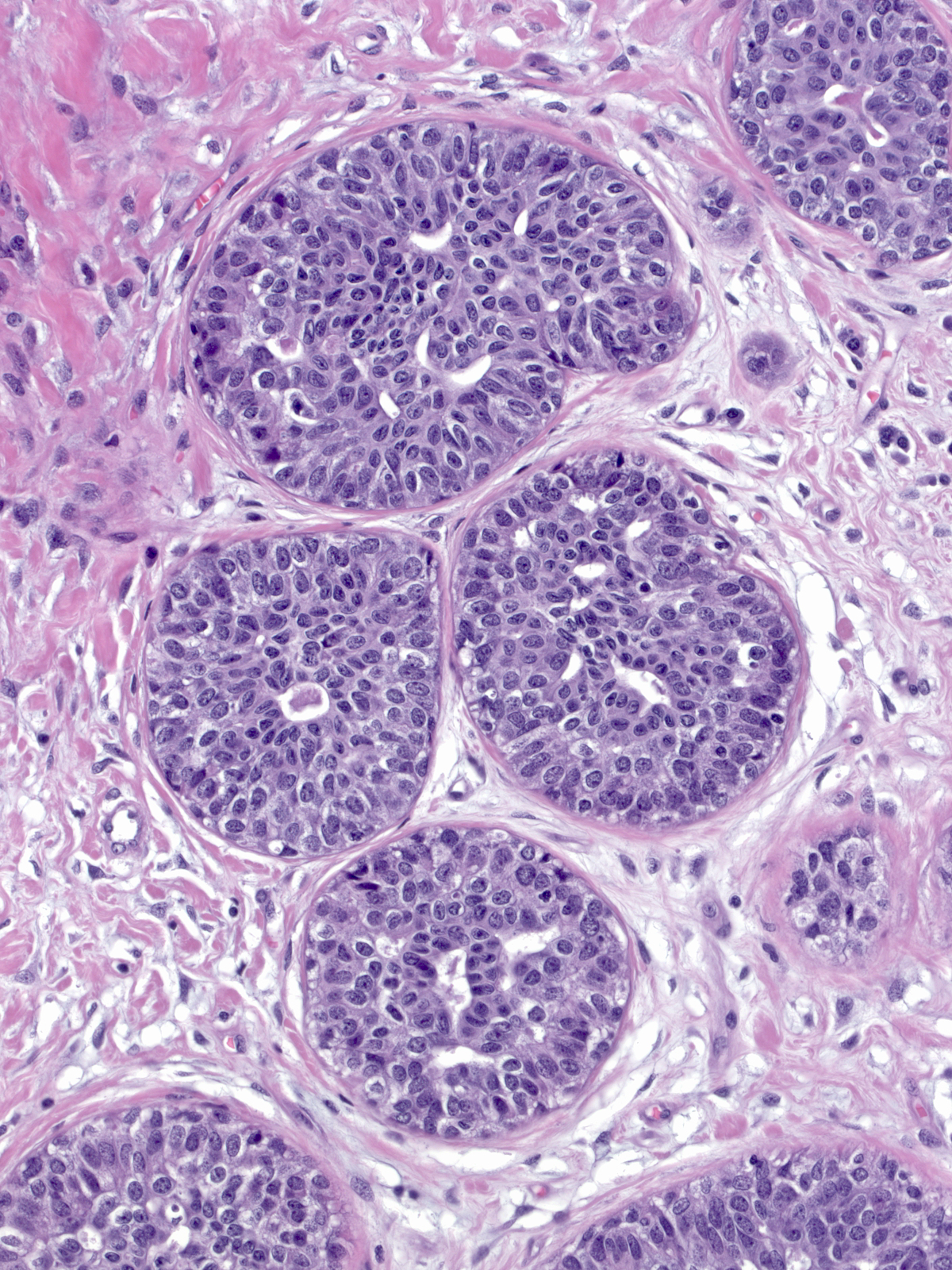 |
 |
 |
tumor) can demonstrate this finding.|24-7 Beta_cat.jpg}}