Difference between revisions of "mgh:cyto-rotation"
| Line 4: | Line 4: | ||
'''Rotation 1 (4 weeks)''' | '''Rotation 1 (4 weeks)''' | ||
Rotation-1 curriculum is intended to be completed during four weeks of AP1. | Rotation-1 curriculum is intended to be completed during four weeks of AP1. | ||
| − | {{:mgh:cyto-1-1}}<br> | + | * {{:mgh:cyto-1-1}}<br> |
{{:mgh:cyto-1-2}}<br> | {{:mgh:cyto-1-2}}<br> | ||
{{:mgh:cyto-1-3}}<br> | {{:mgh:cyto-1-3}}<br> | ||
Revision as of 13:52, July 2, 2020
- Clinical Rotations
- Anatomic Pathology residents spend four weeks on the cytology service during AP1 (rotation-1), and six weeks during AP2 (rotation-2). Each week that you are on the cytology service, you will be assigned to work a faculty member who is on-service, and you will have an opportunity to review and diagnose current cytology cases. Additionally, there are structured learning modules for rotation-1 and rotation-2.
Rotation 1 (4 weeks)
Rotation-1 curriculum is intended to be completed during four weeks of AP1.
Contents
- 1 Lectures
- 2 Cytopathology Laboratory
- 3 Hours of Operation:
- 4 Services Offered
- 5 Critical Values for Cytopathology
- 6 Cytology Specimens
- 7 Specimen Collection
- 8 Specimen Delivery
- 9 Specimen Rejection Policy
- 10 Rapid on site evaluation and interpretation
- 11 FNA Tissue Triage For Radiologically Guided CT/US Guided Biopsies
- 12 Tissue Triage for EBUS Procedures
- 13 Cell classification by morphology
- 14 Morphology of cell response to injury
- 15 Cytomorphology of malignancy
- 16 Lectures
- 17 GYN Cytology: Screening for Cervical Cancer Basic Overview
- 18 Body Cavity Fluids Cases:
- 19 Basic cytomorphology:
- 20 Introduction
- 21 Indications for cytology examination
- 22 Accuracy
- 23 Procuring the CSF sample
- 24 Test platforms/specimen processing and triage
- 25 The CSF Cytology Report
- 26 Basic cytomorphology
- 27 Lecture Slides
- 28 Basic cytomorphology
- 29 Lectures
- 30 Basic cytomorphology
- 31 Lecture slides:
- 32 Basic cytomorphology
- 33 General
- 34 Basic cytomorphology
- 35 Basic cytomorphology
- 36 Basic cytomorphology
- 37 Basic Cytomorphology
- 38 Basic cytomorphology
- 39 Basic cytomorphology
- 40 Basic cytomorphology
- 41 General
- 42 Basic cytomorphology
- 43 General
- 44 GOALS/EXPECTATIONS:
- 45 Regulatory agencies and guidelines
- 46 HPV Testing Methods; additional tests
- 47 Image analysis assisted screening
- 48 Guidelines for management of patients with abnormal cervical cytology results
- 49 Advanced gyn cytomorphology cases:
Lectures
Cytopathology Laboratory
- Location: Warren 125 (6-3980) – Main Cytology Office: supervisor’s office, cytotechnologists’ stations, cytopathology fellow desks (4-1422), secretaries and multi-headed microscope
- Warren 113 (4-1424) – Cytology Specimen Preparation Room: specimen drop off
- Wang 270-FNA exam room (4-1077)
- Wang 290- FNA waiting room on ACC-2
- Staff:
- Director of Cytopathology (Technical Supervisor) - Martha B. Pitman, MD (Warren 105C)
- Director of Fine Needle Aspiration Service: Amy Ly, MD (Warren 105F)
- Technical Director (General Supervisor)- Brenda Sweeney, MS, SCT(ASCP)MB
- Technical Specialist – Ron Arpin, MS, SCT(ASCP)
- Cytopathologists:
- W. Stephen Black-Schaffer M.D
- Elena Brachtel, M.D.
- Ivan Chebib, MD
- William Faquin M.D.
- Joseph Misdraji M.D.
- Rosemary Tambouret M.D.
- David Wilbur, MD
- Cytotechnologists - Peter Brown, Heather Grant, Diane Kuebler, Marilyn Nutter, Mary Pinheiro-Rego, Lisa Ring, and Caitlin Eno
- CLA Secretaries – Bernadette Femino and Diane White
- Lab Preparatory Technologists - Ernest Li, Shirley
Hours of Operation:
- The Cytopathology Laboratory is open Monday through Friday:
- For reports and information: 8:30 a.m. - 5:00 p.m.
- For specimen preparation: 7:30 a.m. – 5:00 p.m.
- After Hours Policy
- Fine Needle Aspirations are not performed by the pathologist service after hours or on weekends under any circumstances.
- Rarely an urgent cytology specimen requires a rapid evaluation during off-hours. This is the case only if the rapid interpretation will affect current patient care. Once the chief resident establishes the medical necessity of the rapid interpretation, the cytopathologist should be called.
- The chief resident has a list of the cytopathology attendings' home telephone numbers. Since there is no on-call rotation for such circumstances, call the attendings in the listed order until one is contacted. The cytopathology staff member will then contact the cytotechnologist to prepare the specimen if necessary.
- Certain cytology specimens such as CSF and pleural effusions are often divided for evaluation by various clinical labs, including cytology. The clinical pathology labs are staffed 24 hours a day and often result such specimens before the cytology lab. If a rush result is requested when the cytology lab is not open, the resident can check if the clinical lab result is available. An example is a spinal tap in a patient with suspected meningeal carcinomatosis. The hematology analysis is often ready first.
Services Offered
- Diagnostic interpretation of cytology specimens:
- All cases are screened by cytotechnologists. Except for negative cervical smears that can be directly signed out by the cytotechnologist, all cases are passed on to the cytopathologist for interpretation, diagnosis and final sign out
- Fine needle aspiration biopsy (FNAB) service:
- The FNAB is a minimally invasive technique to cytologically sample mass lesions. FNABs are performed by cytopathologists at the request of clinicians on patients who have a superficial mass. Pathologists most often use palpation to guide the needle, but have access to ultrasound to guide the FNA for thyroid FNAs, and for more ill-defined masses or for those inadequately productive by palpation guidance. Masses in visceral organs must be sampled by radiologists under imaging guidance. The service is offered Monday through Friday from 9 a.m. to 4 p.m. by appointment (in advance or same day space provided) with the cytopathology secretary (617-726-3980). The procedure is usually performed in the FNAB Clinic, Wang 270. If necessary, the FNAB can be performed in the clinician’s office or in the OR. All in-patient FNABs are performed in their room.
- Rapid Evaluation and Interpretation (Rapids):
- The procedure is akin to the frozen section for surgical pathology. Rapids may be requested by the ordering physician on any specimen sent to the cytology lab. Most often however Rapid On-Site Evaluations (ROSE) are performed. Currently, the services requesting ROSE at MGH include Thoracic Interventional Radiology (Blake 2), Gastroenterology (endoscopic ultrasound (EUS) FNA (Blake 4), Pulmonologist or Thoracic Surgeon performed endobronchial ultrasound guided (EBUS) FNA (Blake 3 Frozen Section Lab) and Pathologist performed FNA (Wang 2). See below for further details.
Critical Values for Cytopathology
- An unexpected diagnosis of malignancy
- Any other clinically significant and time-sensitive finding which was unexpected or unsuspected (as determined by clinical information provided)
- Any significant discrepancy between permanent section and frozen section diagnosis/ interpretation
- An addendum or an amendment report, which provides information significantly different from that rendered in the original report
- Bacteria or fungi in CSF cytology in immunocompromised or immunocompetent patients
- Pneumocystis, fungi or viral cytopathic changes in bronchoalveolar lavage (BAL), bronchial washing or brush cytology specimens in immunocompromised or immunocompetent patients
- Acid-fast bacilli in immunocompromised or immunocompetent patients
- Fungi in any FNA from immunocompromised patients
- Herpes in Pap smears of near term pregnant
- To document notification, insert Coded Comment: “NOTIFY”[F8}
- To flag the case as a critical value case, check the
- Retrieval Flag: CVR [critical value report]
- GYN cases: staff/reflex tab
- Non-GYN case: QA tab
Cytology Specimens
Specimen types:
- Liquid-based preparations (cervical sample dispersed immediately in alcohol).
- SurePath (BD Tripath)
- ThinPrep (Hologic Cytyc)
- Conventional smears (cervical scrap/brush directly applied to glass slide)
- Fluids: included in this category are:
- Body fluids containing cells exfoliated in situ (urine, pleural effusion, ascitic fluid, CSF, sputum, cyst fluids),
- Fluids that were created by washing an epithelial surface with saline and re-aspirating the washed fluid to recover the sloughed cells (pelvic washing, bronchial washing, bronchial-alveolar lavage (BAL), gastrointestinal washing)
- Needle rinses from fine needle aspiration procedures
- Rinses from brush specimens.
- Brushings: An epithelial surface can be brushed during a procedure (usually bronchoscopy or endoscopy of the gastrointestinal or pancreatobiliary tracts) and the material on the brush is either smeared onto the glass slide and fixed or the brush is rinsed into CytoRich Red for LBC processing immediately fixed in a jar of 95% ethanol.
- Fine needle core biopsies: During radiologist-guided FNABs using imaging, the radiologist may elect to also obtain a thin core biopsy which is fixed immediately in formalin and processed as paraffin-embedded biopsy. Since the biopsy is often small, if additional studies are anticipated at the time of processing, a request for blanks on glue may be made by writing CYT X on the surgical pathology requisition sheet where X is the number of blanks desired for ancillary studies (e.g. CYT 4, CYT6, etc).
- Touch preps: A cytology slide can be made by gently touching a surgical biopsy specimen to a glass slide followed by rapid fixation of the slide for staining. This commonly performed by the radiologists during interventional procedures. Touch preps can also be made in the frozen section lab to enhance evaluation of intraoperative tissue analysis.
- Cell blocks: The residua of fluid specimens can be centrifuged after the initial cytology preparation is made in order to harvest the sediment.
Specimen Collection
Cytology test orders are only accepted from individuals authorized to do so in accordance with law and regulation.
- Gyn specimens (Pap smears):
- Conventional smears are fixed in 95% alcohol in a jar supplied by the cytology lab. For liquid based preparations, the sample (taken by broom, brush or spatula) is placed in the appropriate container, 24% ethanol for SurePath (sampling device remains in vial) and 50% methanol for ThinPrep (TP). The TP vial for gyn specimens has a pink label. Include last menstrual period and information on previous abnormal reports, treatments or biopsies on the requisition when available.
- Missing clinical information may be obtained by Cytopathology personnel from CAS to help resolve cytological evaluations when necessary.
- Non-gyn specimens:
- All specimens smeared on slides (brushings, FNABs, touch preps) should be fixed immediately in 95% ethanol in jars provided by the cytology lab and delivered to Warren 113 as soon as possible. To prevent slides within the jar from sticking together, place a small paper clip on alternating slides.
- All non-smear specimens that are bathed in their own fluid (e.g., urines, body fluids, sputum, CSF, BAL’s) should be fresh and delivered to the laboratory as soon as possible, preferably within one hour. No 24-hour collections are acceptable. Saline needle rinses should also be promptly delivered to the cytology lab. Needle rinses placed in fixative (CytoRich Red or formalin) can tolerate a slight delay.
- All specimens must be labeled with the patient's name and unit number and must be accompanied by a properly completed Cytopathology requisition (form #10299). All pertinent information must be included to ensure accurate cytological evaluation and comply with federal regulations. Missing clinical information may be obtained by Cytopathology personnel from CAS to help resolve cytological evaluations when necessary.
Specimen Delivery
- Between 7:30 a.m. and 5:00 p.m. (Monday through Friday), specimens should be delivered to the cytopreparatory laboratory area (Warren 113). When the laboratory is closed, specimens should be taken to the Core lab on Gray 5 between the hours of 5 p.m.-7:30 a.m. Monday-Friday and on weekends/holidays.
- Verbal Orders:
- MGH Department of Pathology Verbal Add on Test Request Form must be used for all verbal orders and is available on the pathology intranet at: http://intranet.massgeneral.org/pathology/documents/Dept%20ADD%20on%20Form%20%20FINAL%20Oct.29%2703.pdf
Specimen Rejection Policy
Specimens received in the Cytopathology Laboratory having identification information that cannot be verified will not be processed. Two patient identifiers must be provided and verified as correct before laboratory specimens will be processed or tested. Both container or slides and requisition must be labeled. The labels must contain the patient’s full name and/or medical record number and/or date of birth. Identifiers must match one another. Specimens that are not readily replaced and have identification discrepancies will be processed upon completion of a Specimen Release form and corrected requisition by an individual authorized to take such responsibility. (See cytology manual for complete procedure).
Rapid on site evaluation and interpretation
- Rapid interpretations are performed on most FNABs performed by the pathologist or other physicians (radiologist, gastroenterologist, pulmonologist, surgeon) in order to guide the procedure. The rapid is intended to assess if the specimen is representative, to determine the need for additional passes and to correctly triage the material for special studies if need be (flow cytometry, cultures, electron microscopy or a request for core biopsy by the radiologist). A definitive diagnosis is not necessary at the time of rapid interpretation although this may be possible in many cases.
- For radiologically obtained FNABs, a cytotechnologist selects one smear per pass, stains the smear with H&E and evaluates the smear. When the tech estimates that the smear is representative, the pathologist is called to give the rapid interpretation.
- Rapid interpretations of previously submitted specimens or specimens obtained by the clinician will be performed only if the rapid interpretation will affect current patient care.
- Please send ALL specimens from the procedure for each patient to cytology at the same time to avoid delays in processing.
FNA Tissue Triage For Radiologically Guided CT/US Guided Biopsies
- Two passes- 4 smears placed in alcohol
- If there are “worms” of tissue or a clot on the slide, use a needle tip to transfer these to small formalin tubs (Peoplesoft #160435 for ordering) or CytoRich Red preservative (from Cytology lab). Do not attempt to smear the “worms” or clots.
- Rinse the needle in CytoRich Red preservative.
2. If lymphoma suspected or tissue needed for culture, rinse needle in saline from a dedicated FNA.
3. If lesion is not amenable for core biopsy OR contains clots from FNA, perform a dedicated FNA explicitly to obtain additional tissue for cell block; rinse in CytoRich Red and request that a cellblock be made.
4. Biopsy for molecular testing must indicate this need on the Histology requisition form. Tissue to be submitted with Cytology Requisition:
- Direct smears
- Washings
- Brushings
- Needle rinsings in CytoRich Red if no clots in container
- Saline rinse for flow cytometry. Contact flow cytometry for a supply of RPMI preservative (6-8487) if there is potential for delay in specimen processing, e.g. a late Friday afternoon procedure.
5. For FNABs performed by the pathologist, slides are stained according to the individual preference of the cytopathologist (H&E, modified Pap stain, toluidine blue or WG).
- Place core biopsy directly into formalin tub or transfer to formalin before submitting to cytology lab. Do not add cores to CytoRich Red preservative.
2. Tissue to be submitted with Histology Requisition (provide clinical information, especially if molecular studies desired):
- Core biopsy in formalin
- Needle rinsings for cellblock if clots placed in solution
3. All tissue with cytology can be sent to the cytology lab for processing with their respective requisitions. Cores only without smears should be submitted to Blake 3 for accessioning.
4. Tissue for culture is sent directly to the microbiology lab.
Tissue Triage for EBUS Procedures
- Cut the end off of the bronchial brush and place in a tube of CytoRich Red solution, also provided by the cytology lab
- If a rapid interpretation is needed of the brushing specimen, make one direct smear on a non-frosted slide and immediately place the slide in a jar of 95% ethanol which is provided by the cytology lab (call 6-3980 for supplies).
- Collect washing/lavage fluid and submit fresh to the cytology lab.
- Express the material near the label end of a non-frosted slide, smear and place in 95% ethanol.
- If there are “worms” of tissue or a clot on the slide, use a needle tip to transfer these to small formalin tubs (Peoplesoft #160435 for ordering). If formalin is not available, these samples can be place in CytoRich Red preservative solution.
- Do not attempt to smear the “worms” or clots.
- Perform a dedicated FNA explicitly to obtain additional tissue for cell block; Express tissue directly into formalin container.
- If lymphoma is suspected, please do a dedicated FNA to obtain tissue for flow cytometry. This tissue should be placed in sterile saline or RPMI preservative (contact flow cytometry for a supply, 6-8487) if there is potential for delay in specimen processing, e.g. a late Friday afternoon procedure.
- If there is a question of infection, please obtain a sample to be sent directly to microbiology.
Core biopsy should be placed in a separate formalin tub. The two formalin tubs are submitted with one histology requisition with two specimens: A. “cellblock” and B. “core”
- Biopsy for molecular testing must indicate this need on the Histology requisition form.
Warning: Display title "1-2 Cytomorphology: Basic Concepts" overrides earlier display title "1-1 Cytopathology Personnel, Procedures and Policies".
Cell classification by morphology
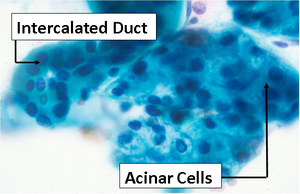
- Cytology diagnosis depends on the appearance of individual cells usually devoid of architectural detail or extracellular matrix. Because the cells are analyzed at much higher magnification than takes place for histology, you need to shift your focus to details overlooked in histologic exams.
- Nuclear morphology provides information on the level of cellular activity while the cytoplasm provides clues to the cell differentiation or cell type.
- Cell shape will vary in cytology samples depending on how the sample was obtained. Exfoliated cells suspended in liquid fixative often have a round shape. Cells that have been plucked from their tissue either by abrasion (brushing, scraping) or fine needle aspiration may retain their native shape (rectangular glandular cells, polygonal squamous cells).
- Epithelial cells
- The quality of the cytoplasm offers clues as to cell origin: cytoplasm of glandular cells is pale staining with one vacuole or many micro vacuoles containing glandular secretions; squamous cells usually have a more dense, uniformly stained cytoplasm and may show a linear quality surrounding the nucleus. Nuclei are generally “euchromic” that is the chromatin is of a normal intensity. In cytology preparations from women, the inactive X chromosome may be seen as a small dense dot on sitting on the nuclear membrane, known as the Barr body.
- Glandular cells
 |
 |
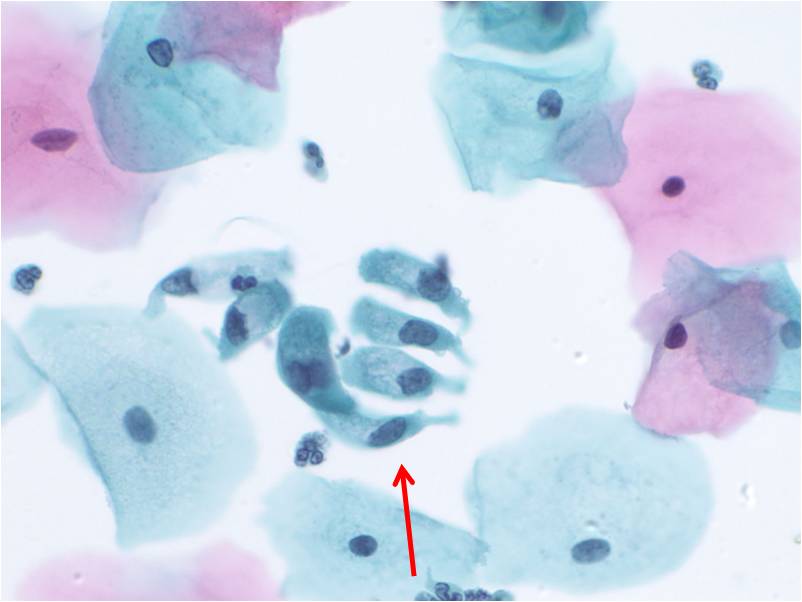
When single, glandular cells have a rectangular or square shape. The cytoplasm is delicate and the nucleus tends to be round with finely distributed (open or vesicular) chromatin. A small nucleolus may be present. The nucleus tends to be present at the end of cytoplasm nearest the prior attachement to the basement membrane. This site of attachement may appear as a cytoplasmic tail where the cell was detached from the basement membrane.Glandular cells in groups can appear as:
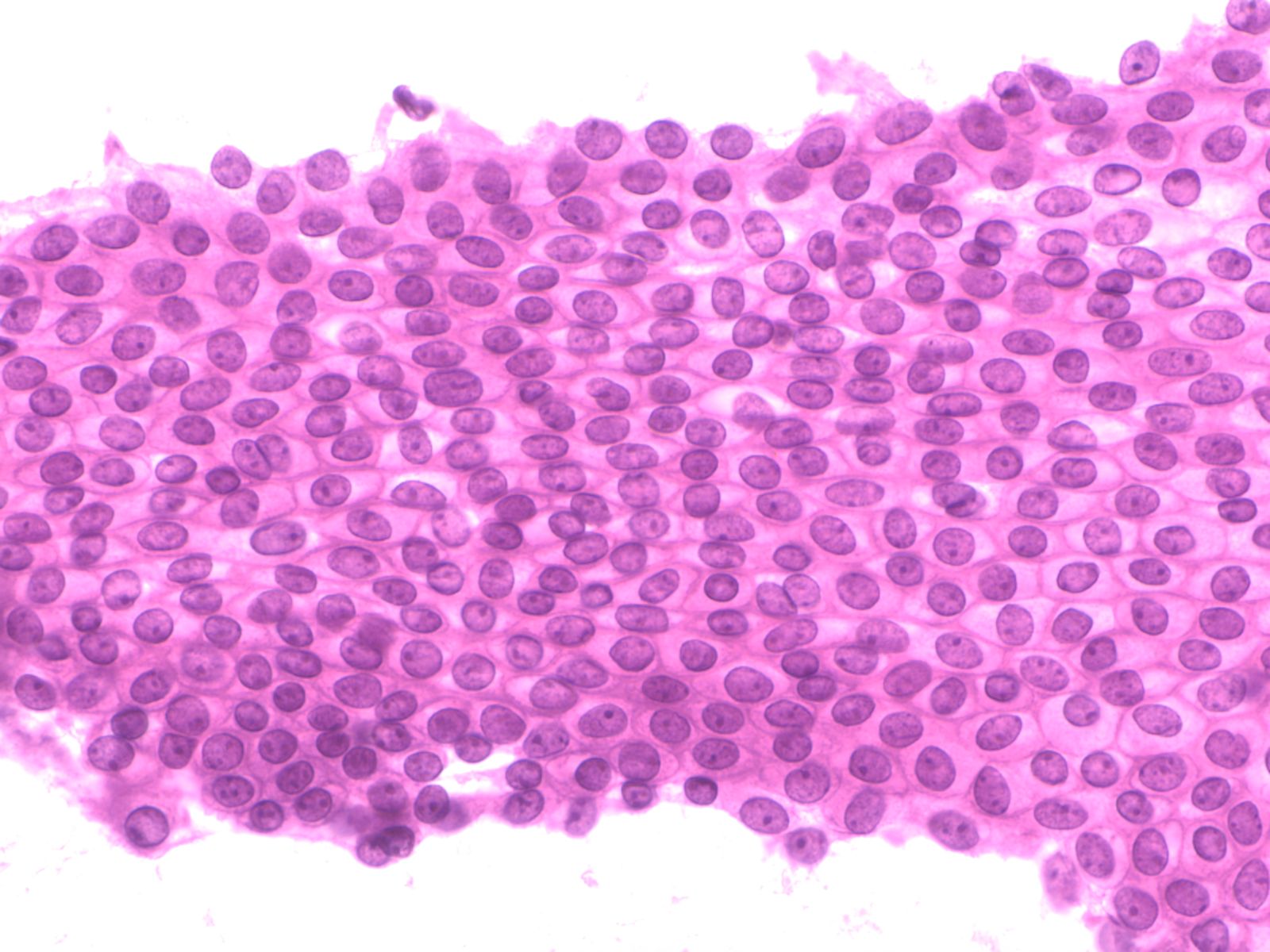
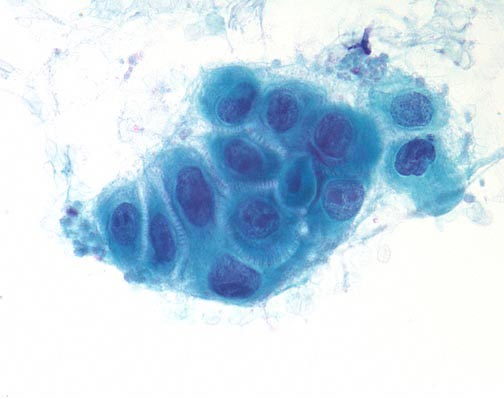
1. Flat sheets with uniform placement of cells in a “honey comb” arrangement similar to a bee hive. By shifting the plane of focus on a glandular sheet, either the mucin-filled cytoplasmic pole or the nuclear pole will be in focus.
 |
 |
|
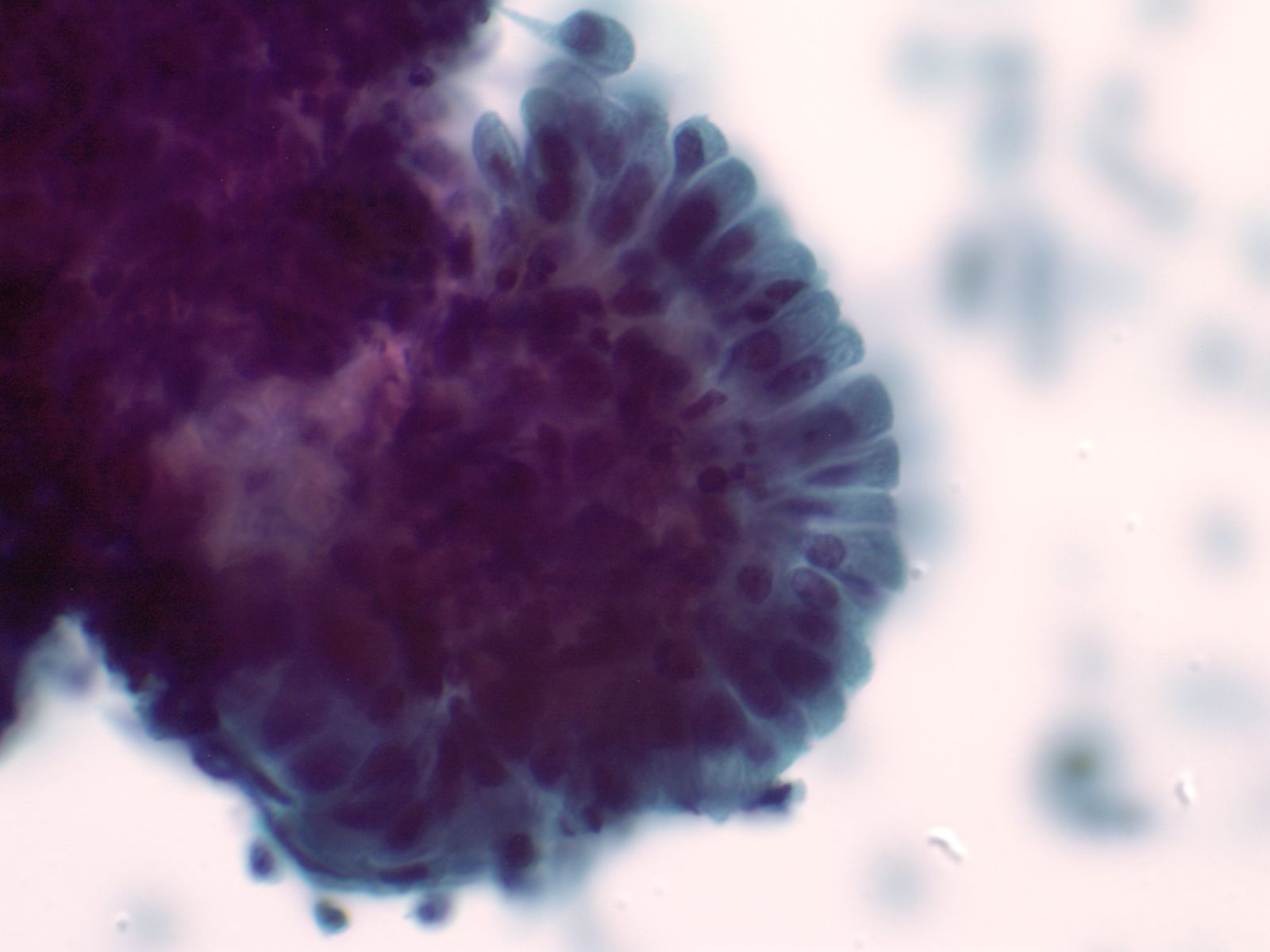
2. Aligned groups of rectangular columnar cells are said to have a picket fence arrangement. The columnar cells may have a row of cilia attached to a terminal bar at one short end of the cell.
 |
 |
|
3. Clustered groups or three dimensional groups of cells with rounded borders often with visible cytoplasmic vacuoles may be abradedor shed from hyperplastic epithelia. Glandular lumens may be visible.
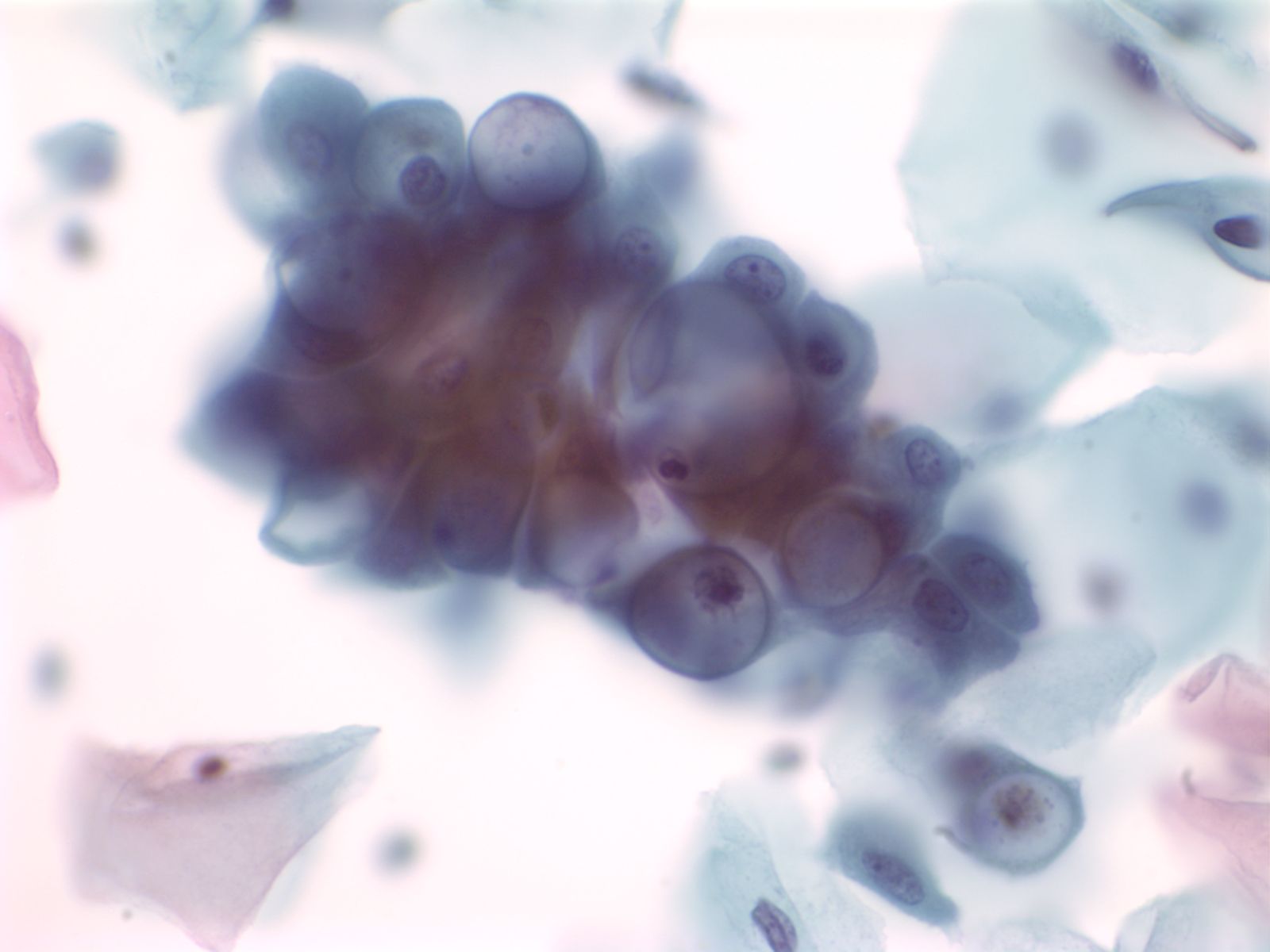 |
||
Squamous Cells
4. Squamous cells when single usually appear as flat polygonal cells. The size of squamous cells varies according to the layer of origin within the epithelium and other factors such as hormone stimulation. The Papanicolaou stain differentially colors the cytoplasm according to the stage of keratinization: younger cells appear more blue-green, more fully keratinized cells turn pink and finally appear orange.
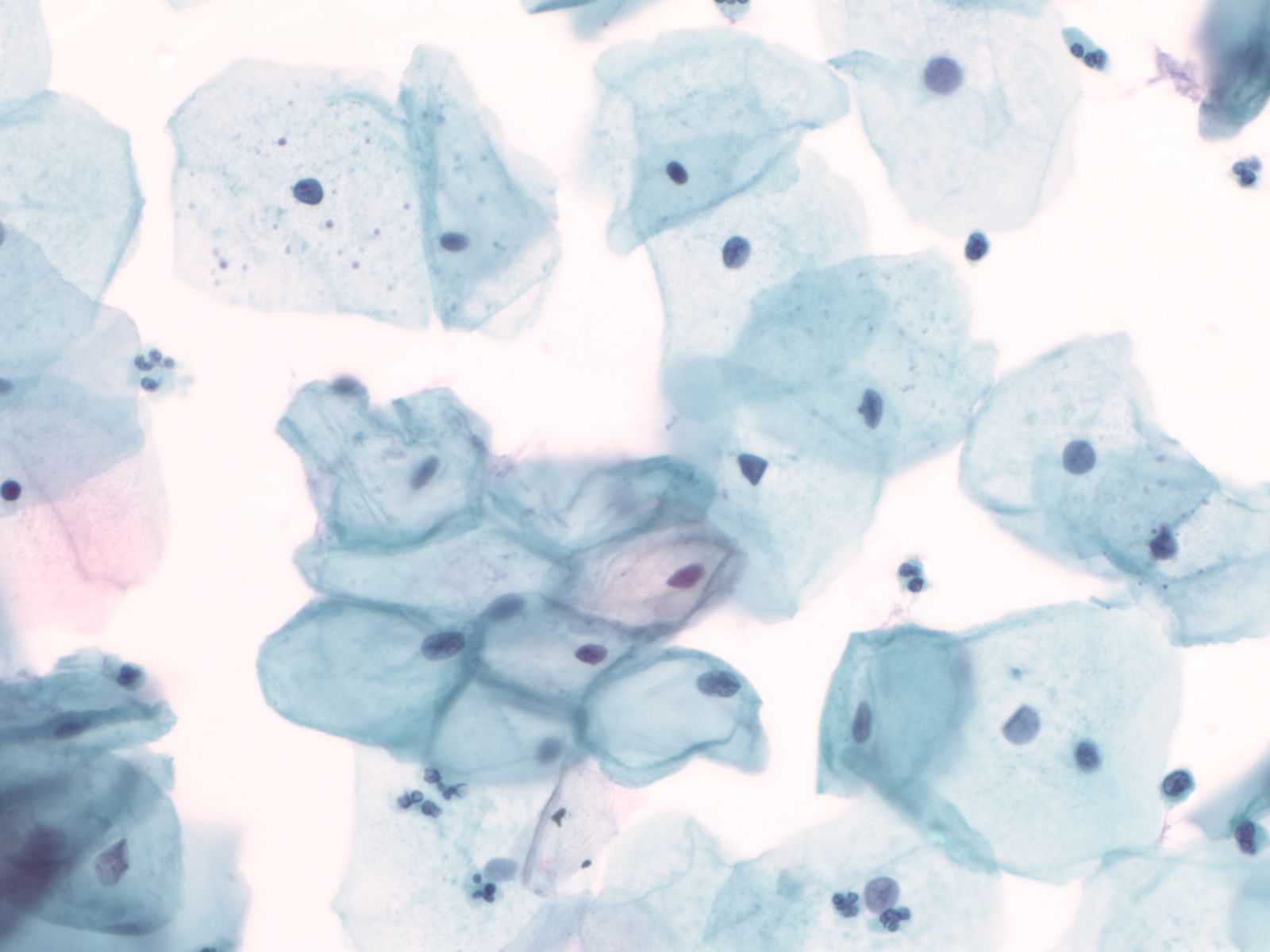 |
 |
The nuclei of the squamous cells vary from larger and round with fine (open) chromatin in less mature cells to pyknotic (dense, dark) and small in superficial cells to a complete absence of nuclei in anucleate squames.
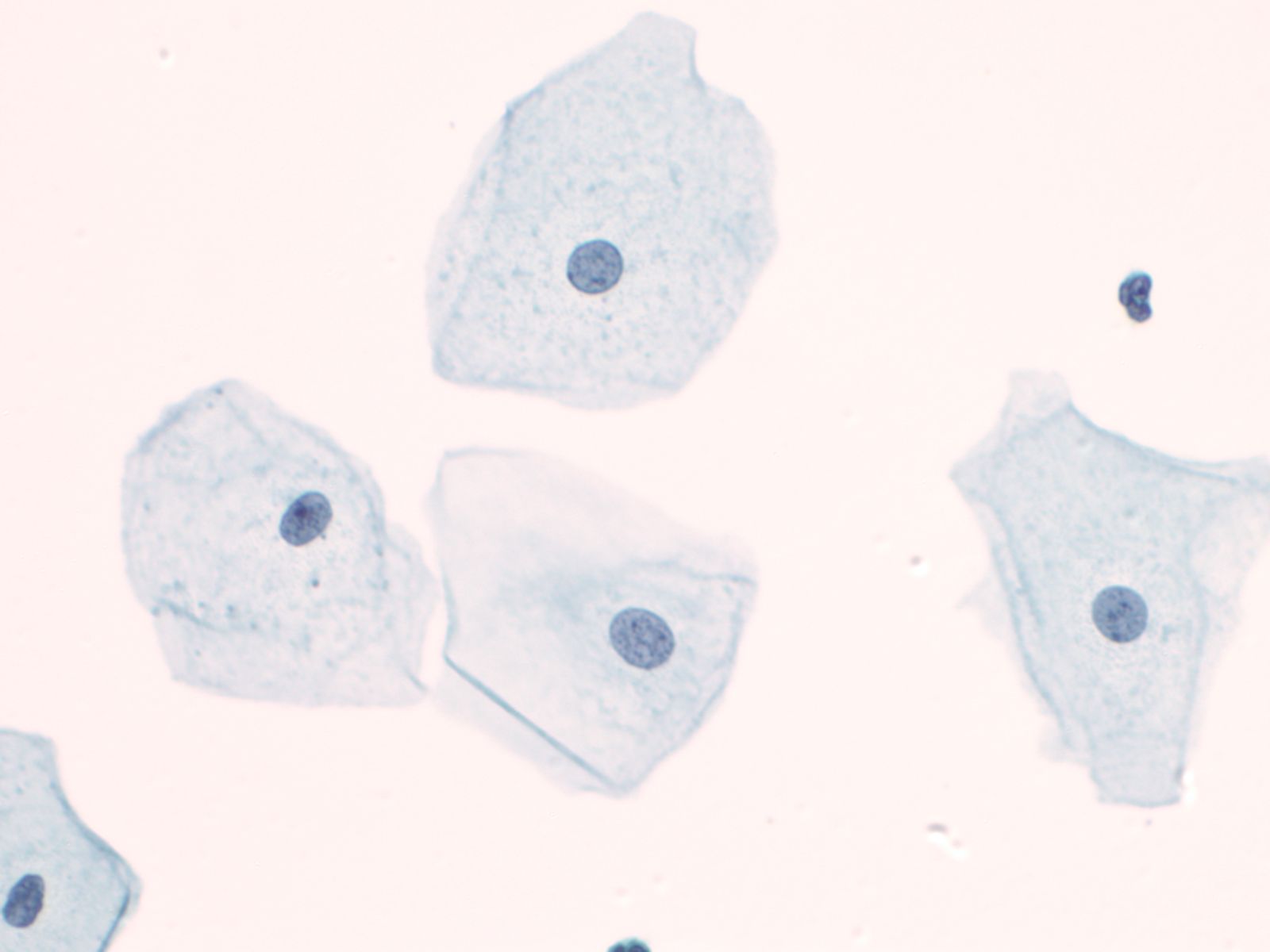 |
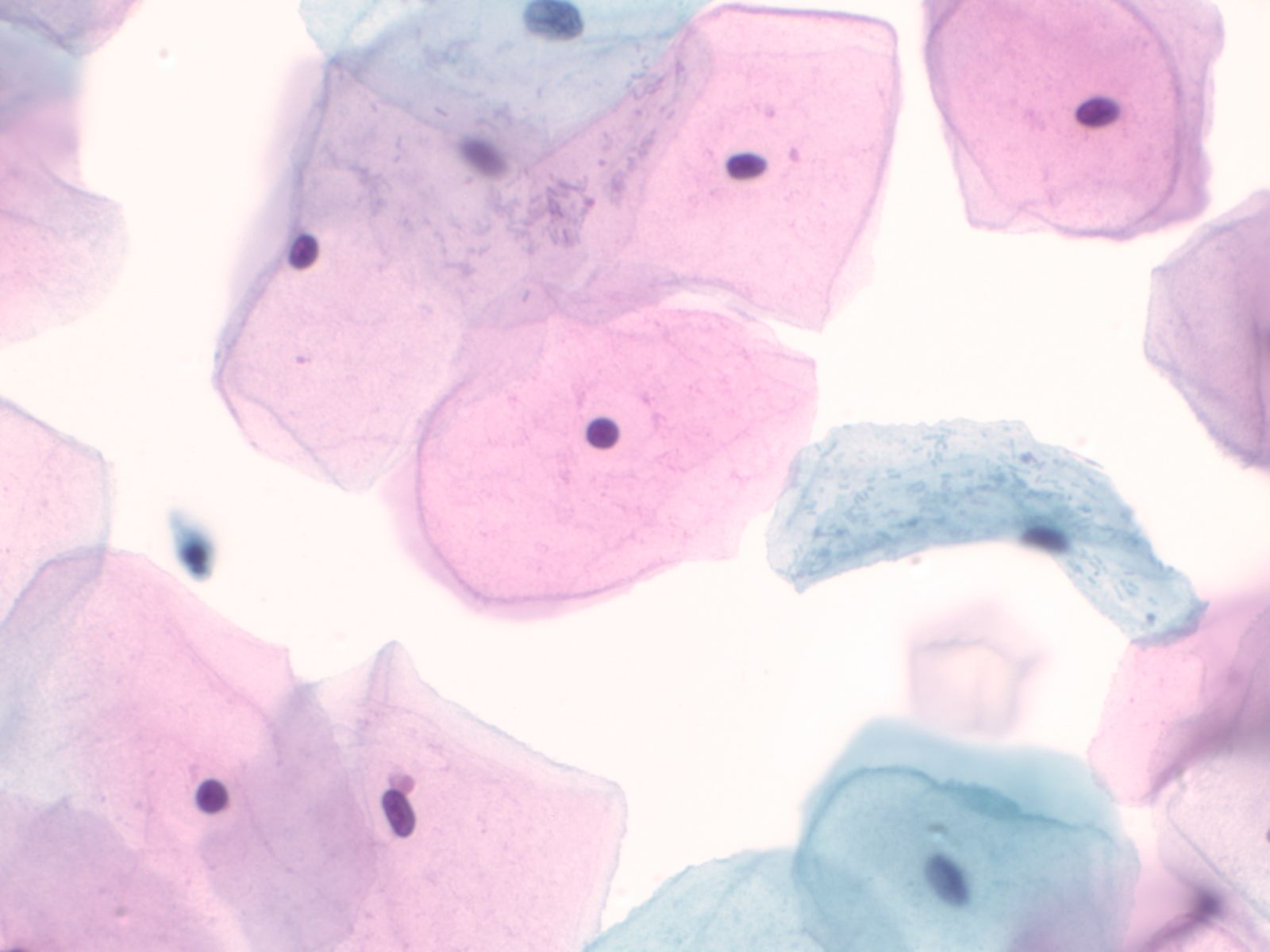 |
 |
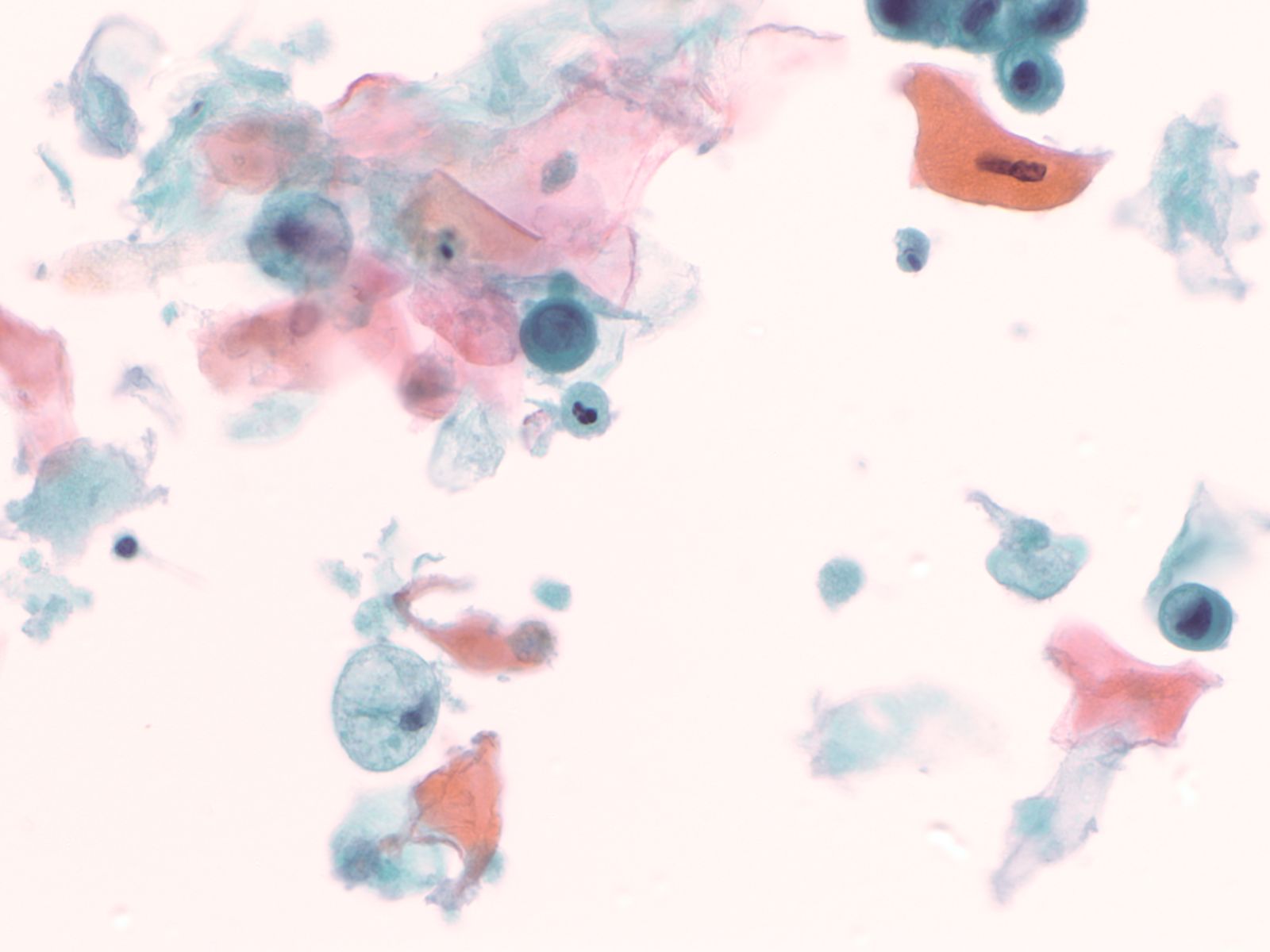
Squamous cells in groups often appear flattened; intercellular junctions can be identified.
 |
||
Mesothelial cells
- These are cuboidal cells that occur in sheets, or when shed in body cavity fluids as rounded cells usually with a dense perinuclear area and paler periphery due to microvilli. When juxtaposed one to another, an intercellular space is visible due to the microvilli.
Mesenchymal cells other than adipocytes often have a spindled or elongate shape. Striations may be seen in skeletal muscle cells
Leukocytes are rounded cells; nuclear shape provides a clue to the cell type: bilobed eosinophils (granules not always obvious), poly lobed neutrophil, reniform shaped macrophage.
Cytoplasmic and extracellular pigment:
- Melanin – brown, dusty to granular on Pap, deep blue with Giemsa
- Hemosiderin – coarsely brown on Pap
- Hematoidin (an intermediate breakdown product of hemoglobin) - yellow
- Bile – deep golden yellow
Morphology of cell response to injury
- Apoptosis
- Necrosis
- Reactive nuclear and cytoplasmic changes:
- Nuclear enlargement B12 deficiency
- Mitoses
- Enlarged nucleoli
- Multinucleation
- Cell enlargement
- Cytoplasmic vacuolization and polychromasia
Cytomorphology of malignancy
Nucleus Multiple nuclear abnormalities occur in malignant cells. No every malignant tumor will show all features and a critical number of features (although there is no magic number) need to be identified before one can say with confidence “this is a malignant cell”. Listed below are the most common changes:
- Nuclear enlargement
- Increased nuclear to cytoplasmic ratio
- Nuclear membrane irregularity
- Chromatin abnormalities: hyperchromasia, coarse irregular chromatin clumping, hypochromasia
- Abnormal nucleoli: excessively large, irregularly shaped, multiple
- Intranuclear inclusions
- Nuclear grooves or creases
- Abnormal mitoses
Cytoplasm and cell arrangement
- Nuclear abnormalities are most critical to the cytologic diagnosis of malignancy, but cytoplasmic and architectural features also occur.
- Depending on the degree of differentiation the malignant cell will vary from near normal in size to extremely large. The malignant cells may vary in size among themselves (anisocytosis).
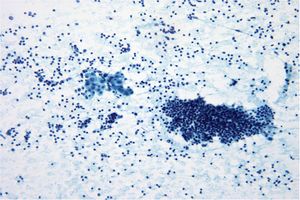
- Orderly cell arrangement is often lost in malignancy, such as the “drunken honeycomb” pattern seen in ductal carcinoma of the pancreas.
- Loss of cell to cell adhesion often occurs in carcinoma, especially in adenocarcinoma. Paradoxically in some squamous cell carcinomas the malignant squamous cells may be seen in large cohesive groups while abraded normal squamous cells occur as single cells.
- Necrosis (also known as tumor “diathesis”) may be apparent on cytology samples as anuclear cellular debris and fibrin present as a film in the background of smears or as material clinging to cell groups in liquid based cytology preparations.
Cytology service page
Warning: Display title "1-3 Gyn Cytology Basics" overrides earlier display title "1-2 Cytomorphology: Basic Concepts".
Lectures
- Lecture: Normal cervical cytology by Dr. D. Wilbur
- Lecture: HPV testing, QA/QC by Dr. D. Wilbur
- Test platforms/specimen processing and triage
- GYN samples are submitted for processing as liquid based specimens. Samples are collected using one of the recommended SurePath collection devices (broom or combination brush/spatula). After the sample is obtained, the head of the collection device is snapped off and put into the SurePath preservative vial. If HPV testing was requested, the test will be done using the tube that was produced during the processing of the SurePath vial.
GYN Cytology: Screening for Cervical Cancer Basic Overview
- HPV and cervical cancer: an overview
- Guidelines for cervical cancer screening
- Obtaining cervical cytology sample (Pap smear)
- Primary screening method
- Bethesda system for reporting gyn cytology and cytology report structure including adequacy
- Test platform for gyn cytology: instruments, stains
- Resident Training in Primary Screening: See Marilyn Nutter
- All residents are to screen 20 Pap tests as primary screeners.
Cytology service page
Warning: Display title "1-4 Body Cavity Fluids" overrides earlier display title "1-3 Gyn Cytology Basics".
Body Cavity Fluids Cases:
Ivan Chebib MD, Amy Ly MD, Ron Arpin SCT
- Indications for cytology examination
by Amy Ly, M.D.
The pleural, pericardial, and peritoneal cavities are lined by serosa, which is a simple layer of mesothelial cells. Under normal conditions, these cavities contain only a small amount of fluid which allows adjacent serosal surfaces to move over each other with low resistance during normal organ activities (e.g. breathing, heartbeats, peristalsis). In disease states, a greater amount of fluid accumulates and is called an effusion. Effusions may be characterized clinically as transudative or exudative. Transudates result from unbalanced hydrostatic and oncotic pressures. Exudates result from injury to the mesothelium, which is commonly caused by malignant tumors that have spread to serosal surfaces and/or malignant mesothelioma that originates in the serosa.
Detection of serosal malignancy by cytologic exam is more sensitive than by blind biopsy (58%-71% compared with 45%). Cytology sensitivity is further increased by 2%-38% if more than one sample is examined. However, the false negative rate is still significant. If cytology is negative but there is high suspicion for pleural malignancy, the patient can undergo thoracoscopy for further evaluation.
The specificity of cytologic effusion evaluation is very high: the false positive rate is <1%. False positive and false suspicious diagnoses are mainly due to reactive mesothelial cells that appear atypical.
Gynecologic and non-gynecologic malignancies involving the peritoneal serosal surfaces may not produce an effusion or be associated with lesions visible by gross inspection intraoperatively. In such cases, the peritoneal cavity may be evaluated by “peritoneal washing,” which is part of a cancer staging procedure. Peritoneal washings may also be used to exclude occult malignancy in patients undergoing laparoscopy or laparotomy for presumed benign gynecologic conditions and in women with BRCA1/2 mutations undergoing risk reducing salpingo-oophorectomy. Peritoneal washing may be potentially utilized to monitor a patient's response to adjuvant treatment for cancer.
Peritoneal washings that are positive for malignancy are associated with worse prognosis in patients with ovarian and fallopian tube cancers. Positive washings alone impact the surgical stage of only 3-5% of women with gynecologic cancers, but may be the only evidence of metastasis to the peritoneum for some patients. Peritoneal washing results are included in the International Federation of Gynecology and Obstetrics ovarian and fallopian tube cancer staging algorithm. The prognostic utility of this test for endometrial and other gynecologic cancers is unclear at this time.
There is a significant false-negative rate with peritoneal washings. 23-86% of patients with biopsy proven peritoneal metastasis have no evidence of disease in their washings by cytologic exam. The high false negative rate may be partly due to poor distribution of fluid within peritoneal cavities that have been affected by adhesions. False positive diagnoses are not common (<5% of cases), and are usually due to proliferative mesothelial cells with reactive changes and associated psammoma bodies, and endometriosis.
- Procuring the specimen
by Amy Ly, M.D.
Effusion specimens are obtained by inserting a needle into the pleural space (thoracentesis), pericardial space (pericardiocentesis), and peritoneal cavity (abdominal paracentesis). Peritoneal fluid is usually obtained through the abdominal wall, however in women it may also be aspirated from the cul-de-sac through the vagina (cold to centesis). Effusions may also be collected during thoracic, abdominal, or cardiac surgery. Removing this excess fluid may be performed for diagnostic purposes (submitted for pathology evaluation) or therapeutic purposes (to alleviate symptoms such as dyspnea and heart failure). Large volumes (several liters or more) of abdominal fluid may be drained safely. However, pleural fluid that is rapidly removed in large quantities may rarely be complicated by reexpansion pulmonary edema. This condition is fatal in up to 20% of cases and tends to involve younger patients with a long duration of lung collapse who experience rapid lung reexpansion upon thoracentesis.
The effusion is collected in sterile containers and sent unfixed to the laboratory. Specimen collection into glass containers causes rapid clotting, which is undesirable as this causes dispersion of cells and makes it more difficult to evaluate them. To prevent clotting, collect fluids into heparinized bottles containing 3 units of heparin per milliliter of capacity. If heparinized bottles are not available, the heparin should be placed into the container before the fluid is drained. Store fluids at 4°C until the time of slide preparation. Effusions are robust specimens and may be refrigerated for > 2 weeks without compromising cellular morphology or antigenicity for immunostains because the effusion itself nourishes the cells within it. However, specimens involved by malignancies with high cellular turnover (e.g. Burkitt lymphoma) should be prepared as soon as possible.
Peritoneal washes are obtained intraoperatively. The surgeon evacuates any pre-existing peritoneal fluid and submits it separately for cytologic examination. Sterile saline (50-200 mL) is instilled into multiple areas, usually the pelvis, the right and left paracolic gutters, and the undersurface of the diaphragm. A repeat washing or rinsing action is used to abrade cells from the serosal surfaces into the saline. The saline is then pooled into a single collection and heparinized. There is no advantage to submitting washings from different sites separately. The specimen should be delivered to the laboratory unfixed and stored at 4°C until slides can be prepared. If there will be a significant delay before slide preparation, an equal volume of 50% ethanol can be added to preserve the specimen.
- Test platforms/specimen processing and triage
by Amy Ly, M.D.
To make slides from an effusion, the first steps are to agitate the fluid to evenly disperse the cells and then to centrifuge up to 50 mL of the fluid. The supernatant is discarded and the pellet is used to prepare smears, cytocentrifuge preparations (Cytospins), or thin-layer preparations (e.g. ThinPrep, SurePath). The slides are usually alcohol fixed but if a lymphoproliferative disorder is suspected, air dried Cytospins are helpful. Slides are stained with a Papanicolaou or Romanowsky type stain. Residual fluid is set aside in case additional slides or other preparations/tests such as cell block, flow cytometry, and molecular studies are needed.
Cell blocks may be prepared from fluids by coagulating the sediment into a compact mass with plasma and thrombin, wrapping the sediment in filter paper, placing in a cassette, and processed in the manner of histologic sections (fixing in formalin, embedding in paraffin, cutting, and staining with H&E). Clots that are already present in the fluid because it was not heparinized should be placed in cassettes for processing as cell blocks. The addition of a cell block to a smear/Cytospin/thin-layer slide increases sensitivity for the detection of malignancy. Cell block sections are useful for special and immunohistochemical stains. Cell block sections are also convenient for morphologic comparison with histologic sections because the tissues have been processed in an identical manner.
To prepare slides from a peritoneal washing, the specimen is thoroughly mixed and 50 mL of fluid is centrifuged. The supernatant is discarded and the pellet can be used to prepare smears, cytocentrifuge preparations (Cytospins), or thin-layer preparations (e.g. ThinPrep, SurePath). The remaining material or a separately centrifuged cell pellet can also be fixed in 10% formalin and processed as a cell block, employing histologic methods of processing, paraffin embedding, cutting, and H&E staining. Cell block sections are useful for morphologic comparison to the patient's resected neoplasm and for performing special and immunohistochemical stains.
- Reporting and terminology
by Amy Ly, M.D.
There are no established criteria for adequacy of effusion specimens. Cytologic diagnosis of fluids utilizes the following categories: “no malignant cells identified,” “atypical” (low suspicion for malignancy), “suspicious” (high suspicion for malignancy), and “positive for malignant cells.” The diagnosis of malignancy is semi-quantitative and semi-qualitative. “No malignant cells identified” and “positive for malignancy cells” are self-explanatory unequivocal diagnoses. Indeterminate categories of “atypical” and “suspicious for malignancy” are used when abnormal cells are present, but are too poorly preserved or too few in number to render a definitive diagnosis of malignancy. Approximately 5% of specimens are diagnosed as “suspicious.” In such cases, the effusion will usually re-accumulate if there is a serosal malignancy; the next sample may contain evidence of malignancy.
Adequacy criteria for peritoneal washing cytologic specimens have not been established, but there should be at least a few groups of well-preserved benign mesothelial cells present before concluding that the specimen is adequate for evaluation and negative for malignant cells. Specimens with malignant cells are always adequate. Results of peritoneal washing cytology are commonly reported as negative, atypical, suspicious, or positive for malignant cells. Atypical and suspicious interpretations should be avoided if possible because they are not helpful for treatment decision-making. Usually, only an unequivocally positive diagnosis is used for staging purposes, and atypical and suspicious results are considered to be negative results. Equivocal cytology washing cases may be resolved by comparing morphology a current corresponding resection specimen.
Basic cytomorphology:
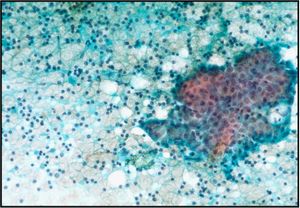
- Sheets of benign mesothelial cells are often smaller than 12 cells, but may sometimes be composed of upwards of 50 cells
- In these photomicrographs, the even dispersal of uniform cells, with regular nuclei, delicate nuclear membranes and small round nucleoli signal the benign nature of these cells
- Under conditions of an inflammatory process, mesothelial cells are increased in number, can exhibit a wide range of sizes, and may be multinucleated
- The keys to diagnosis involve (1) applying individual criteria of benignity and (2) establishing the presence of an uninterrupted continuum of sizes from small to very large
- Note enlarged nuclei, small multiple nucleoli, and spaces between adjacent cells, so called "windows"
- Inflammatory cells are present in the background
- Like pleural effusion, mesothelial cells in peritoneal effusions may exhibit a range of cell sizes
- Mesothelial cells may be admixed with inflammatory cells and histiocytes.
- The key to diagnosing mesothelioma is not identifying a second malignant cell population
- Final determination may require immunocytochemistry or a cell block with immunohistochemistry, electron microscopy, or other specialized techniques
- Individual malignant mesothelial cells exhibit a rim of ruffled, less dense cytoplasm (ectoplasm), surrounding dense cytoplasm around the nucleus (endoplasm)
- Tumor cells may be seen in a background of blood and proteinaceous debris
- Groups of more than 12 cells may be a feature of malignancy.
- High N/C ratio with variability in nuclear size and occasional multi-nucleation confirm the malignant nature of these cells
- Differential diagnoses include adenocarcinoma and mesothelioma
- Fine microscopic features of peripheral cell membranes and intercellular windows may suggest mesothelioma
- Abnormal mitotic figures may be noted with mesothelioma, other malignancies, as well as occasional reactive mesothelial cells in effusions
- Papillary glandular arrangements of the tumor cells
- Prominent nucleoli, vacuolization and mitotic figures
- Distinctions from other sources of adenocarcinoma may be impossible.
- Metastatic ductal carcinoma cells exhibits large irregular nuclei and nucleoli
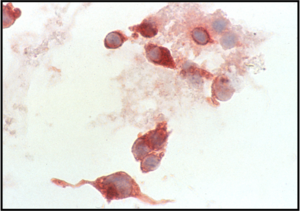
- The classic description of metastatic breast cancer in pleural effusions employs the term "cannonballs" to emphasize the rounded arrangement of tumor cells
- They may have a relatively small nuclear size
- Nuclei are vesicular with prominent nucleoli
- Cytoplasmic vacuoles are uncommon
- A cell block of the cells allows for assay of hormonal receptors or other epithelial markers, such as her-2-neu
- Cells of papillary serous ovarian adenocarcinoma in a pleural effusion represent a discontinuous population of cells
- Their cell and nuclear size is variable
- Increased nuclear to cytoplasmic ratio and cytoplasmic vacuoles are features
- Cells may exist singly or in small acinar groups
- Vigorous peritoneal washes may dislodge microscopic tumor
- Washes are an integral part of staging laparoscopy
- Because of the washing procedure, tumor cells generally come off in three- dimensional cohesive groups and may be admixed with sheets of benign mesothelium
- The tumor cells are easily distinguished by size, malignant characteristics and crowded configurations
- Gastric adenocarcinoma
- Cells with malignant features are present as a distinct population
- Some may exhibit nuclear displacement by a large secretory vacuole, a "signet ring" cell
- Origin from one part of the GI tract over another cannot be easily ascertained
- Cholangiocarcinoma, either from an intra-hepatic source or from an extra-hepatic biliary tree, may look like adenocarcinoma from elsewhere in the GI tract
- By exclusion of other sources through endoscopy, ultrasonography and/orCT imaging, the location may be determined.
- Dyshesive single cells
- Malignant nuclear features, eccentric nuclei
- Range of patterns: small, spindle or epithelioid cells
- Nuclear size variation
- Nuclear pseudoinclusions with bi-, and multinucleation
- Intracytoplasmic dusty brown melanin pigment
- S-100, HMB-45, Melan-A positive (not always)
- Dyshesive single cells
- Open granular chromatin
- Nucleoli based on nuclear membrane in some subtypes
- Nuclear membrane protrusions and irregularity
- Scant cytoplasm in some subtypes (high N/C ratios)
- Lymphoglandular bodies in background
- LCA positive, B or T cell lineage
Cytology service page
Warning: Display title "1-5 Cerebrospinal Fluid (CSF)" overrides earlier display title "1-4 Body Cavity Fluids".
- Ivan Chebib MD, Amy Ly MD, Ron Arpin SCT
- Reading: Cibas 4th Ed. Chapter 6; Bibbo/Wilbur 4th Ed. Chapter 16
- Questions: When you have completed unit 1-5 go to the Assessment tab and answer the question.
- Dr. Tambouret CSF Lecture
- Indications for cytology examination
by Amy Ly, M.D.
The pleural, pericardial, and peritoneal cavities are lined by serosa, which is a simple layer of mesothelial cells. Under normal conditions, these cavities contain only a small amount of fluid which allows adjacent serosal surfaces to move over each other with low resistance during normal organ activities (e.g. breathing, heartbeats, peristalsis). In disease states, a greater amount of fluid accumulates and is called an effusion. Effusions may be characterized clinically as transudative or exudative. Transudates result from unbalanced hydrostatic and oncotic pressures. Exudates result from injury to the mesothelium, which is commonly caused by malignant tumors that have spread to serosal surfaces and/or malignant mesothelioma that originates in the serosa.
Detection of serosal malignancy by cytologic exam is more sensitive than by blind biopsy (58%-71% compared with 45%). Cytology sensitivity is further increased by 2%-38% if more than one sample is examined. However, the false negative rate is still significant. If cytology is negative but there is high suspicion for pleural malignancy, the patient can undergo thoracoscopy for further evaluation.
The specificity of cytologic effusion evaluation is very high: the false positive rate is <1%. False positive and false suspicious diagnoses are mainly due to reactive mesothelial cells that appear atypical.
Gynecologic and non-gynecologic malignancies involving the peritoneal serosal surfaces may not produce an effusion or be associated with lesions visible by gross inspection intraoperatively. In such cases, the peritoneal cavity may be evaluated by “peritoneal washing,” which is part of a cancer staging procedure. Peritoneal washings may also be used to exclude occult malignancy in patients undergoing laparoscopy or laparotomy for presumed benign gynecologic conditions and in women with BRCA1/2 mutations undergoing risk reducing salpingo-oophorectomy. Peritoneal washing may be potentially utilized to monitor a patient's response to adjuvant treatment for cancer.
Peritoneal washings that are positive for malignancy are associated with worse prognosis in patients with ovarian and fallopian tube cancers. Positive washings alone impact the surgical stage of only 3-5% of women with gynecologic cancers, but may be the only evidence of metastasis to the peritoneum for some patients. Peritoneal washing results are included in the International Federation of Gynecology and Obstetrics ovarian and fallopian tube cancer staging algorithm. The prognostic utility of this test for endometrial and other gynecologic cancers is unclear at this time.
There is a significant false-negative rate with peritoneal washings. 23-86% of patients with biopsy proven peritoneal metastasis have no evidence of disease in their washings by cytologic exam. The high false negative rate may be partly due to poor distribution of fluid within peritoneal cavities that have been affected by adhesions. False positive diagnoses are not common (<5% of cases), and are usually due to proliferative mesothelial cells with reactive changes and associated psammoma bodies, and endometriosis.
- Procuring the specimen
by Amy Ly, M.D.
Effusion specimens are obtained by inserting a needle into the pleural space (thoracentesis), pericardial space (pericardiocentesis), and peritoneal cavity (abdominal paracentesis). Peritoneal fluid is usually obtained through the abdominal wall, however in women it may also be aspirated from the cul-de-sac through the vagina (cold to centesis). Effusions may also be collected during thoracic, abdominal, or cardiac surgery. Removing this excess fluid may be performed for diagnostic purposes (submitted for pathology evaluation) or therapeutic purposes (to alleviate symptoms such as dyspnea and heart failure). Large volumes (several liters or more) of abdominal fluid may be drained safely. However, pleural fluid that is rapidly removed in large quantities may rarely be complicated by reexpansion pulmonary edema. This condition is fatal in up to 20% of cases and tends to involve younger patients with a long duration of lung collapse who experience rapid lung reexpansion upon thoracentesis.
The effusion is collected in sterile containers and sent unfixed to the laboratory. Specimen collection into glass containers causes rapid clotting, which is undesirable as this causes dispersion of cells and makes it more difficult to evaluate them. To prevent clotting, collect fluids into heparinized bottles containing 3 units of heparin per milliliter of capacity. If heparinized bottles are not available, the heparin should be placed into the container before the fluid is drained. Store fluids at 4°C until the time of slide preparation. Effusions are robust specimens and may be refrigerated for > 2 weeks without compromising cellular morphology or antigenicity for immunostains because the effusion itself nourishes the cells within it. However, specimens involved by malignancies with high cellular turnover (e.g. Burkitt lymphoma) should be prepared as soon as possible.
Peritoneal washes are obtained intraoperatively. The surgeon evacuates any pre-existing peritoneal fluid and submits it separately for cytologic examination. Sterile saline (50-200 mL) is instilled into multiple areas, usually the pelvis, the right and left paracolic gutters, and the undersurface of the diaphragm. A repeat washing or rinsing action is used to abrade cells from the serosal surfaces into the saline. The saline is then pooled into a single collection and heparinized. There is no advantage to submitting washings from different sites separately. The specimen should be delivered to the laboratory unfixed and stored at 4°C until slides can be prepared. If there will be a significant delay before slide preparation, an equal volume of 50% ethanol can be added to preserve the specimen.
- Test platforms/specimen processing and triage
by Amy Ly, M.D.
To make slides from an effusion, the first steps are to agitate the fluid to evenly disperse the cells and then to centrifuge up to 50 mL of the fluid. The supernatant is discarded and the pellet is used to prepare smears, cytocentrifuge preparations (Cytospins), or thin-layer preparations (e.g. ThinPrep, SurePath). The slides are usually alcohol fixed but if a lymphoproliferative disorder is suspected, air dried Cytospins are helpful. Slides are stained with a Papanicolaou or Romanowsky type stain. Residual fluid is set aside in case additional slides or other preparations/tests such as cell block, flow cytometry, and molecular studies are needed.
Cell blocks may be prepared from fluids by coagulating the sediment into a compact mass with plasma and thrombin, wrapping the sediment in filter paper, placing in a cassette, and processed in the manner of histologic sections (fixing in formalin, embedding in paraffin, cutting, and staining with H&E). Clots that are already present in the fluid because it was not heparinized should be placed in cassettes for processing as cell blocks. The addition of a cell block to a smear/Cytospin/thin-layer slide increases sensitivity for the detection of malignancy. Cell block sections are useful for special and immunohistochemical stains. Cell block sections are also convenient for morphologic comparison with histologic sections because the tissues have been processed in an identical manner.
To prepare slides from a peritoneal washing, the specimen is thoroughly mixed and 50 mL of fluid is centrifuged. The supernatant is discarded and the pellet can be used to prepare smears, cytocentrifuge preparations (Cytospins), or thin-layer preparations (e.g. ThinPrep, SurePath). The remaining material or a separately centrifuged cell pellet can also be fixed in 10% formalin and processed as a cell block, employing histologic methods of processing, paraffin embedding, cutting, and H&E staining. Cell block sections are useful for morphologic comparison to the patient's resected neoplasm and for performing special and immunohistochemical stains.
- Reporting and terminology
by Amy Ly, M.D.
There are no established criteria for adequacy of effusion specimens. Cytologic diagnosis of fluids utilizes the following categories: “no malignant cells identified,” “atypical” (low suspicion for malignancy), “suspicious” (high suspicion for malignancy), and “positive for malignant cells.” The diagnosis of malignancy is semi-quantitative and semi-qualitative. “No malignant cells identified” and “positive for malignancy cells” are self-explanatory unequivocal diagnoses. Indeterminate categories of “atypical” and “suspicious for malignancy” are used when abnormal cells are present, but are too poorly preserved or too few in number to render a definitive diagnosis of malignancy. Approximately 5% of specimens are diagnosed as “suspicious.” In such cases, the effusion will usually re-accumulate if there is a serosal malignancy; the next sample may contain evidence of malignancy.
Adequacy criteria for peritoneal washing cytologic specimens have not been established, but there should be at least a few groups of well-preserved benign mesothelial cells present before concluding that the specimen is adequate for evaluation and negative for malignant cells. Specimens with malignant cells are always adequate. Results of peritoneal washing cytology are commonly reported as negative, atypical, suspicious, or positive for malignant cells. Atypical and suspicious interpretations should be avoided if possible because they are not helpful for treatment decision-making. Usually, only an unequivocally positive diagnosis is used for staging purposes, and atypical and suspicious results are considered to be negative results. Equivocal cytology washing cases may be resolved by comparing morphology a current corresponding resection specimen.
Introduction
CSF is produced by the choroid plexus in lateral, 3rd and 4th venticles by passive filtration and active transport. The CSF circulates through the subarachnoid space from the ventricles to bathe the brain and spinal cord. The Chorioid plexus consists of frond-like villous projections of vessels and pia mater that protrude into the ventricles. Specialized ependymal cells known as choroidal epithelium overlies the villi. The CSF is resorbed in the archanoid villi in the superior sagittal and intracranial venous sinuses and around spinal nerve roots. The arachnoid villi function as one way valves.
Indications for cytology examination
The CSF is examined in many clinical situations. The CSF is submitted for cytology examination usually only when a malignancy is suspected, either metastatic solid tumors or lymphoma/leukemia. Leptomeningeal (LM) metastasis is diagnosed in about 5% of patients with metastatic carcinoma. The tumors most likely to involve the CSF in order of frequency are breast, lung, melanoma, GI tumors and carcinoma of unknown primary. Primary brain tumors can involve the CSF. Forty percent of primary CNS lymphomas will have LM involvement
Accuracy
The sensitivity of CSF cytology for malignancy ranges from 80 to 95%. False-positive results are very rare, but false negative (FN) results are not uncommon. To minimize FN a minimum of 10 cc CSF should be sent to cytology, the sample should be processed promptly and a repeat sample obtained if malignancy suspected but results are negative. One study showed increasing sensitivity with repeat samples of 71% for first, 86% for second, 90% for third and 98% for > 3 samples (Glantz MJ et al. Cancer 1998;82:733).
Procuring the CSF sample
Usually the CSF is obtained by lumbar puncture. Samples may also be obtained from an Ommaya resevoir which consists of a subcutaneous pouch connected to a cannula ending in one of the lateral ventricles.
Test platforms/specimen processing and triage
Currently in the MGH lab two cytospin slides are prepared from a fresh CSF sample. One slide is fixed in 95% ethanol immediately after preparation and stained with Papanicolaou stain. The second is allowed to air dry and then is stained with rapid Giemsa stain used at MGH.
IMG_0737.MOV
The CSF Cytology Report
The results are report as one of four categories: Negative for malignant cells, Atypical (low degree of suspicion for malignancy), Suspicious (a high degree of suspicion for malignancy) or Positive for malignant cells. Over 90% of CSF samples are reported as Negative.
Basic cytomorphology
- Rare lymphocytes, monocytes and PMN's
- Occasionally, ependymal cells, arachnoidal cells and choroid plexus cells are found
- Squamous cells, chondrocytes and red blood cells may be found as contaminants
- Singly distributed, usually monomorphic population of cells with high N:C ratio
- Nuclei are irregular with clumped chromatin
- Macronucleoli may be present
- Mitotic activity may be evident
- Cells are usually singly distributed with occasional loose clusters
- Nuclei are round to oval, centrally or eccentrically located and may be multiple
- Nuclear chromatin is vesicular with eosinophilic macronucleoli
- Coarse, brown melanin granules may be present within the cytoplasm
- Adenocarcinoma - cells are present singly or in small clusters
- Nuclei are irregular, three dimensional and eccentrically located
- Nucleoli are often present
- May be cytoplasmic vacuolization
- Small cell carcinoma - cells are present in small, molded groups
- Nuclei exhibit classic salt and pepper chromatin pattern and may be angular
- Cells have only a scant rim of fragile cytoplasm
Cytology service page
Warning: Display title "1-6 Urine Cytology: R. Tambouret MD, E. Brachtel MD, Ron Arpin SCT" overrides earlier display title "1-5 Cerebrospinal Fluid (CSF)".
Lecture Slides
- Indications for cytology examination
- Procuring the specimen
- Test platforms/specimen processing and triage
- Reporting and terminology
Basic cytomorphology
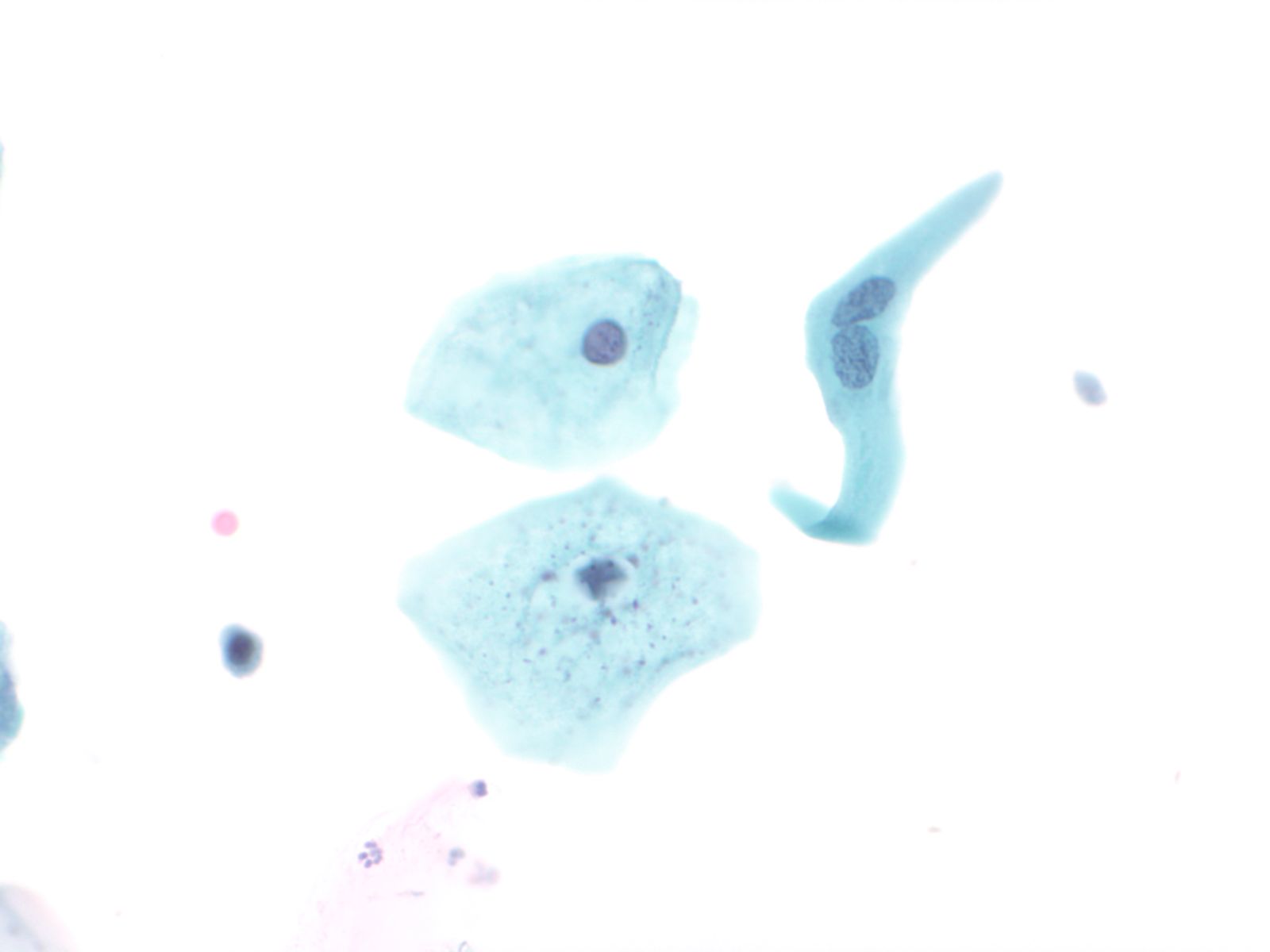
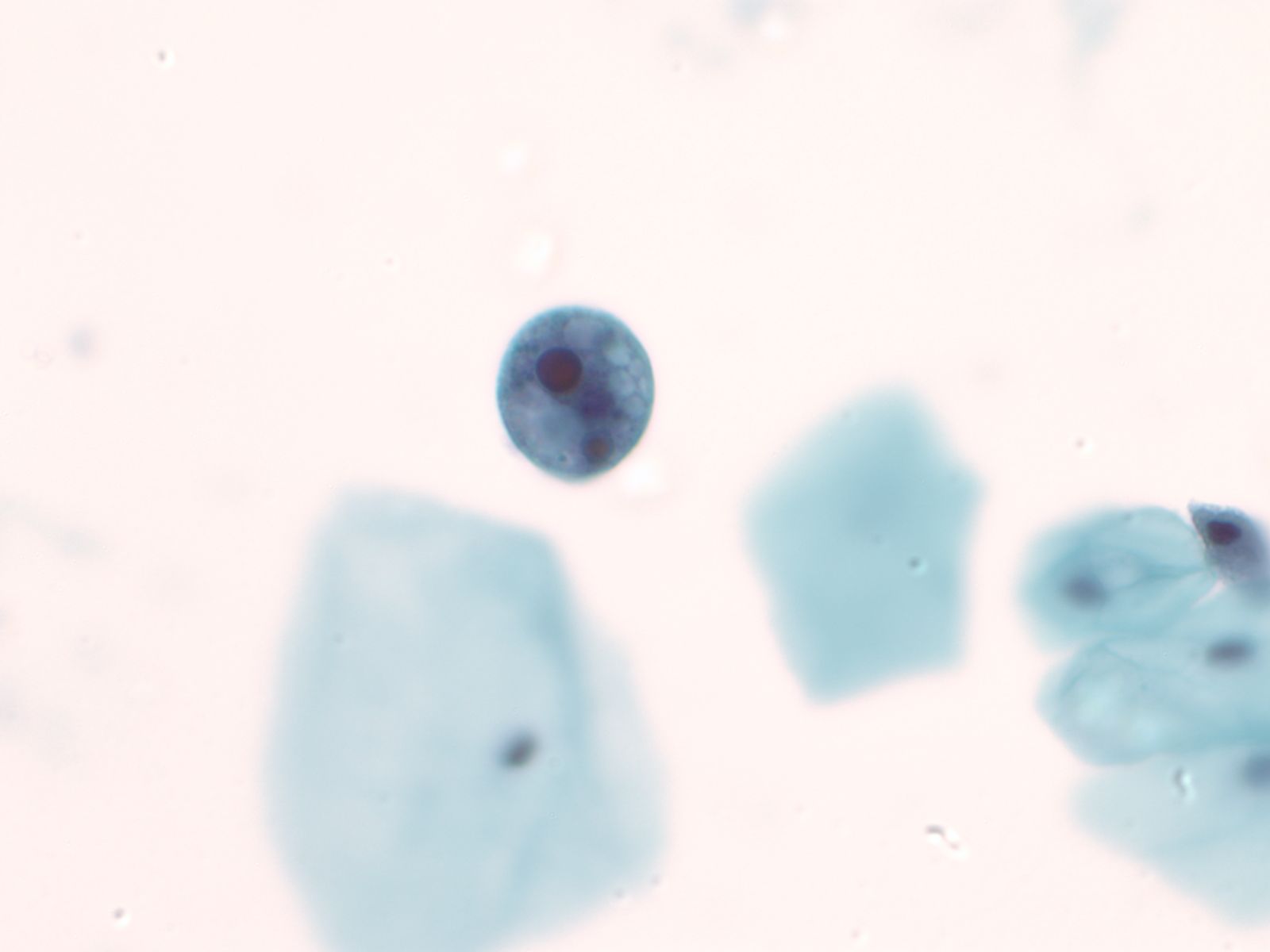
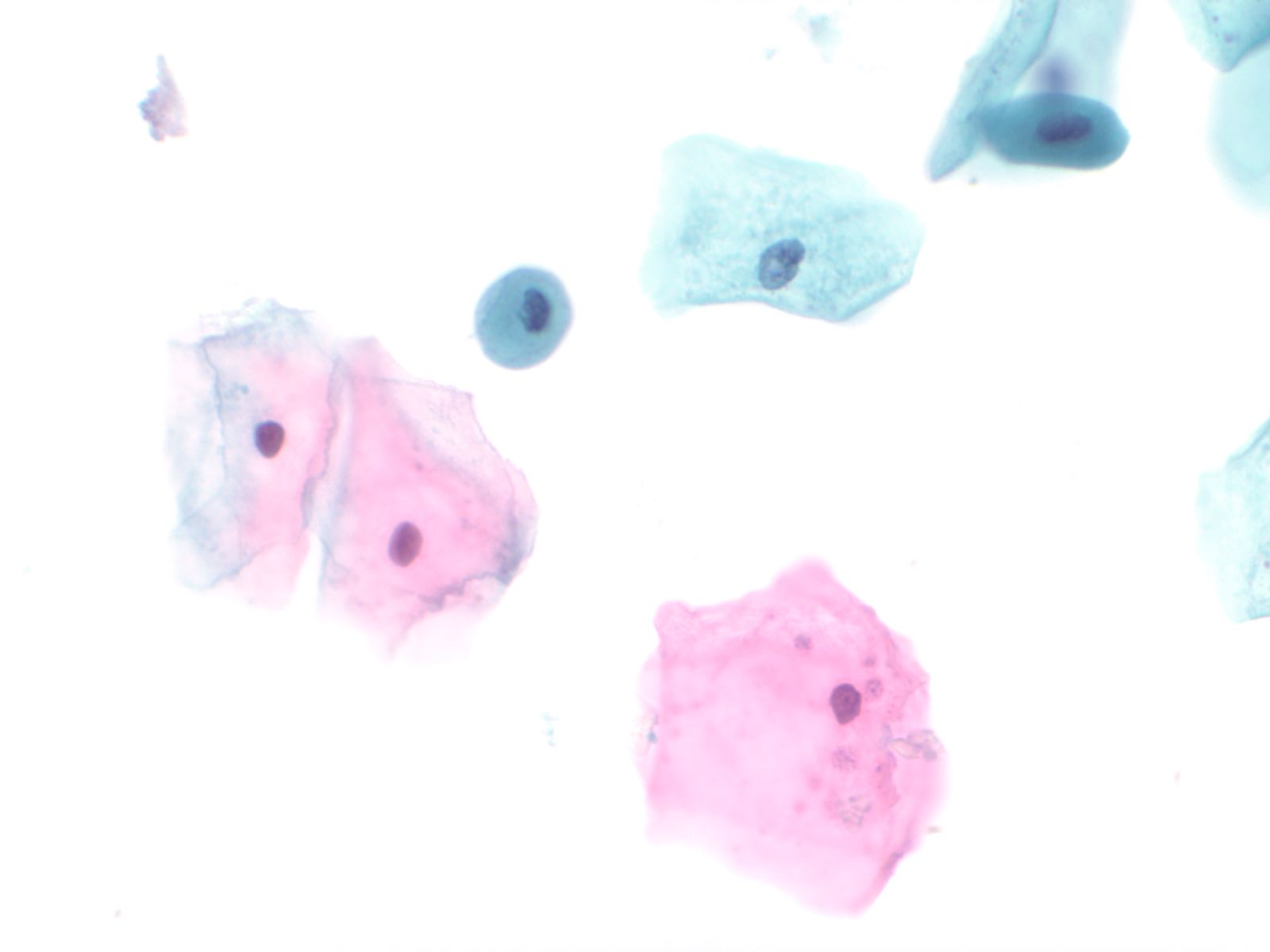
Normal urothelial cells – N13-593
- Normal urothelial cells vary greatly in numbers, sizes, and shape
- Mononuclear urothelial cells are cuboidal, and parabasal-like or polyhedral
- Surface umbrella cells are large, often multinucleated, with convex surface corresponding to the lumen of the bladder
- Cytoplasm blue to grey and occasionally shows fine vacuolization
- Round to ovoid nuclei are centrally located with finely granular chromatin and small nucleoli
- Urothelial cells may show thin cytoplasmic tails (cercariform cells)
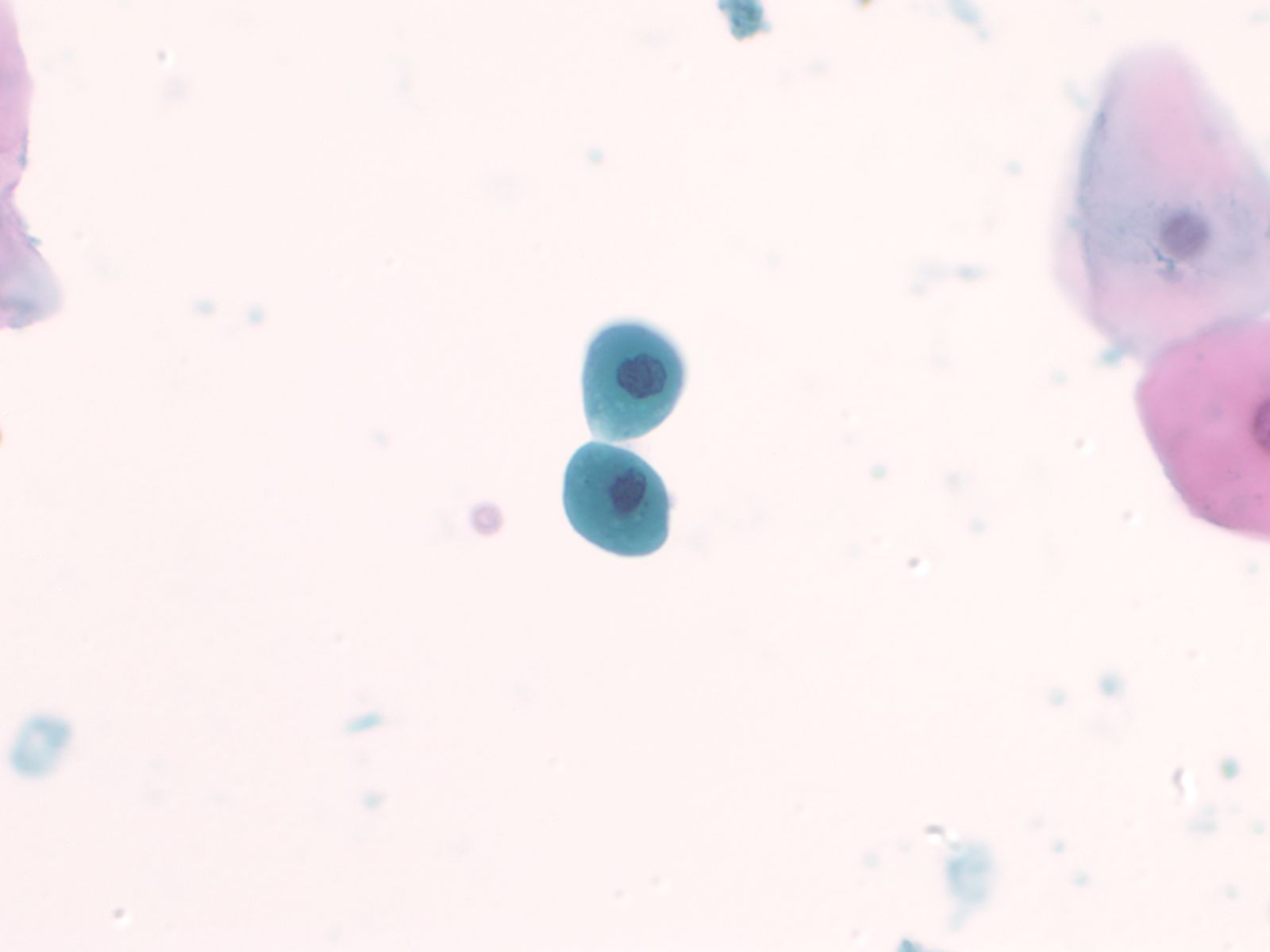 |
 |
 |
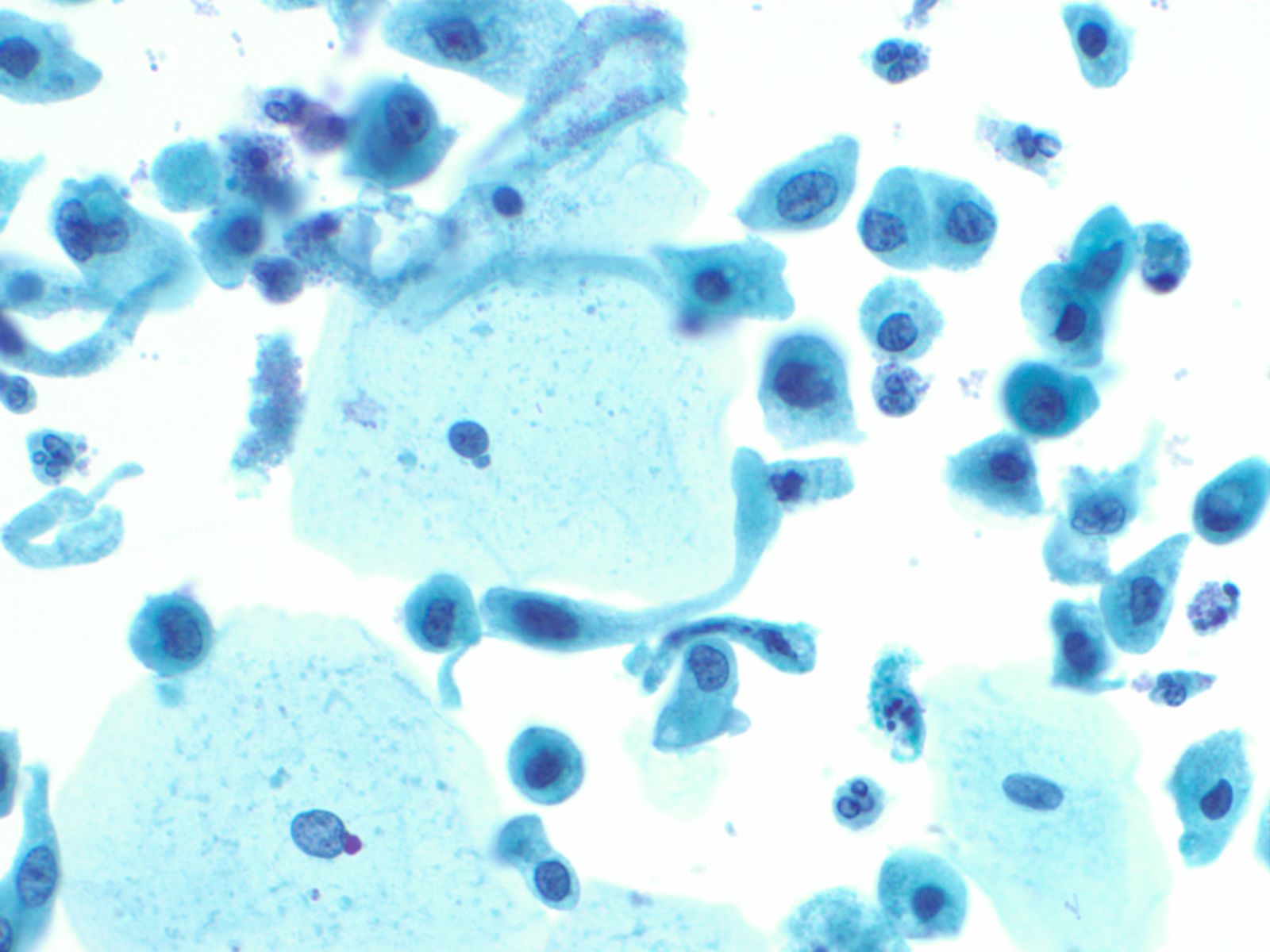 |
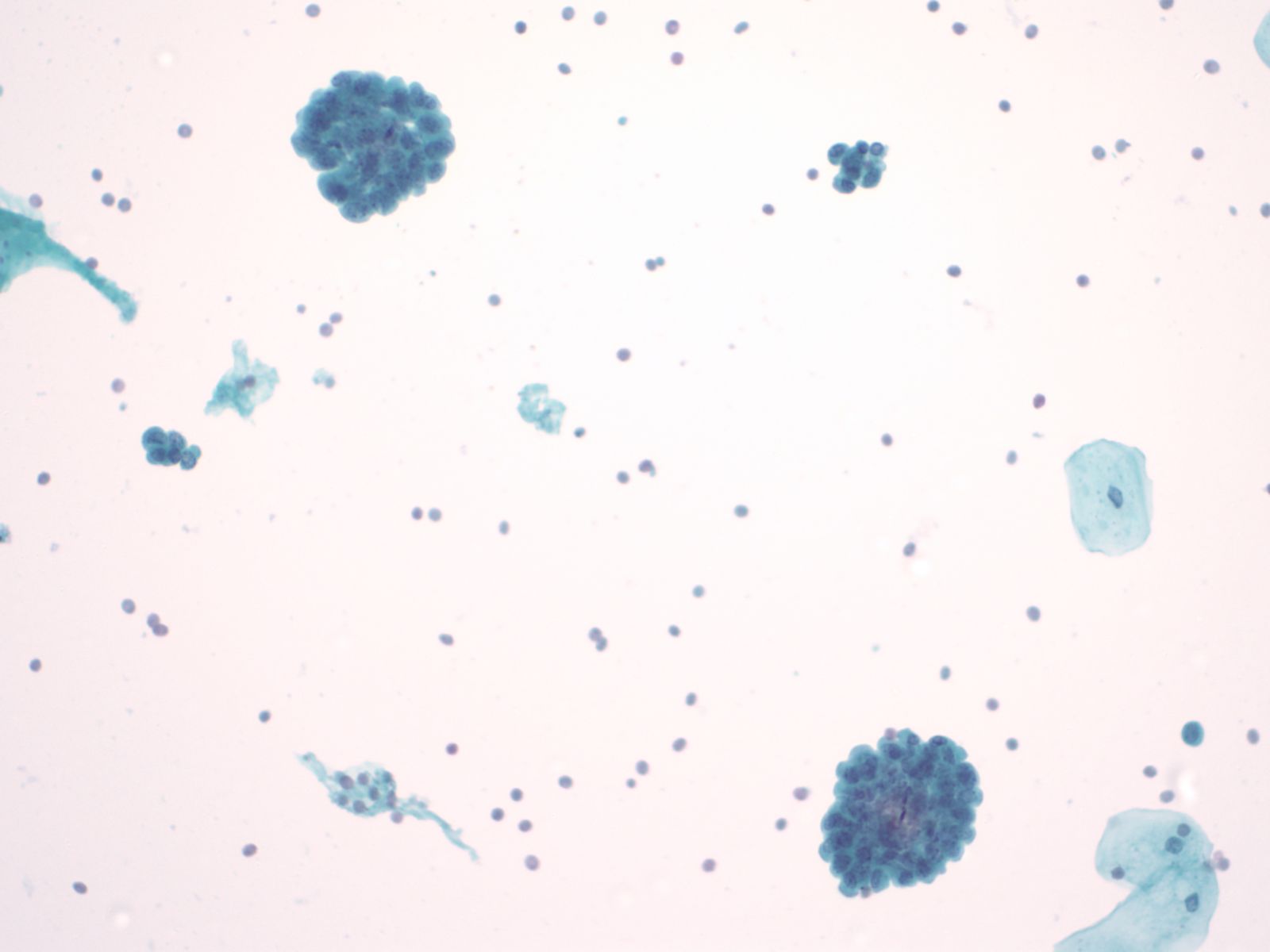
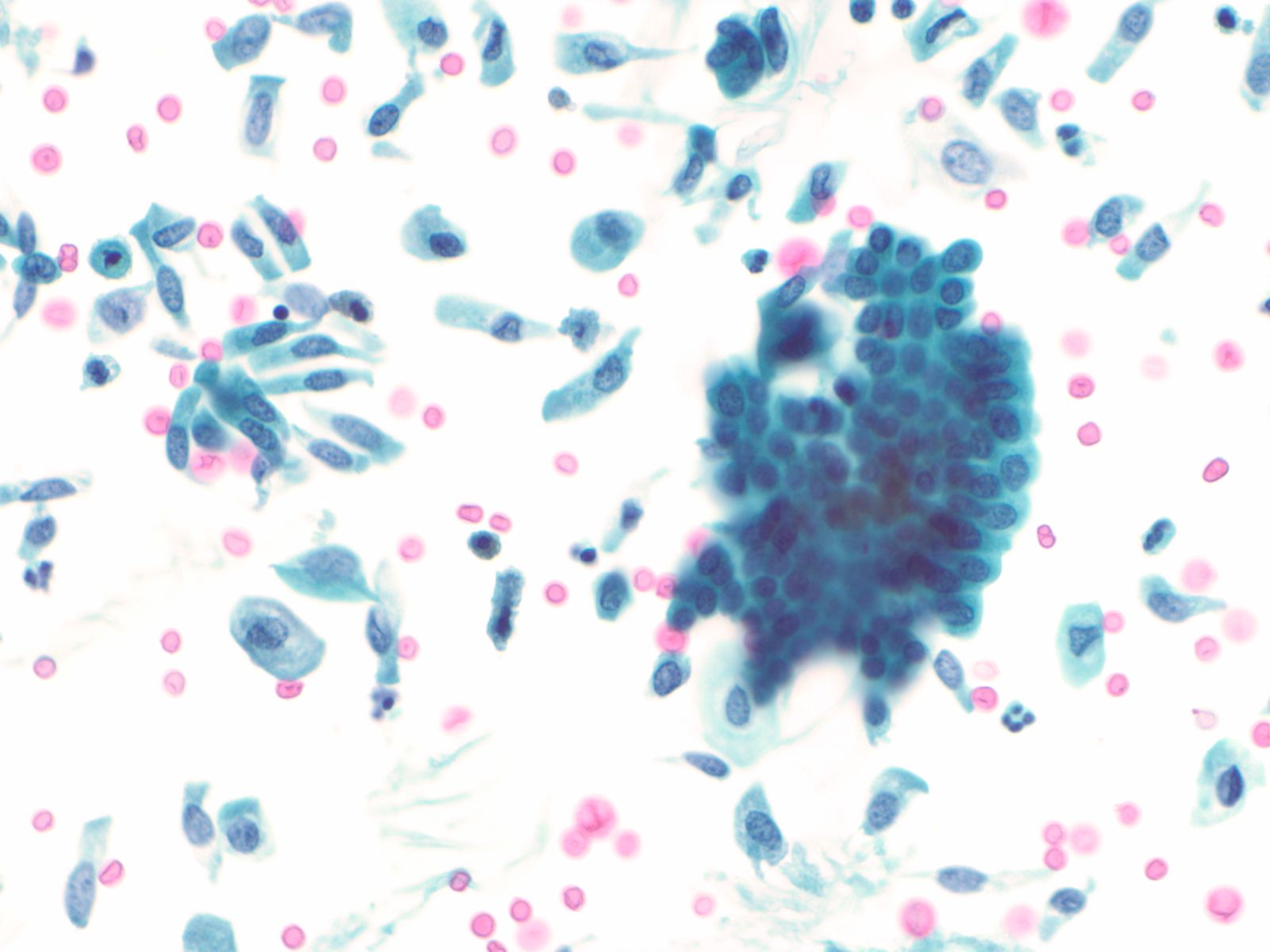
Urothelial clusters and papillary fragments – N13-7330
- Approximately 20% of normal voided urine samples may contain urothelial clusters
- This finding is considerably enhanced in bladder washings, catheterized urine, and brushings due to the propensity of urothelium to exfoliate
- The interpretation of papillary urothelial neoplasm should be made with caution and needs to be correlated with other findings
- Cells in the clusters show nuclei that may appear hyperchromatic or pale and may contain one or more nucleoli
- Occasionally may see a cap of umbrella cells on one side of these clusters, especially in cell groups abraded by instrumentation
 |
||
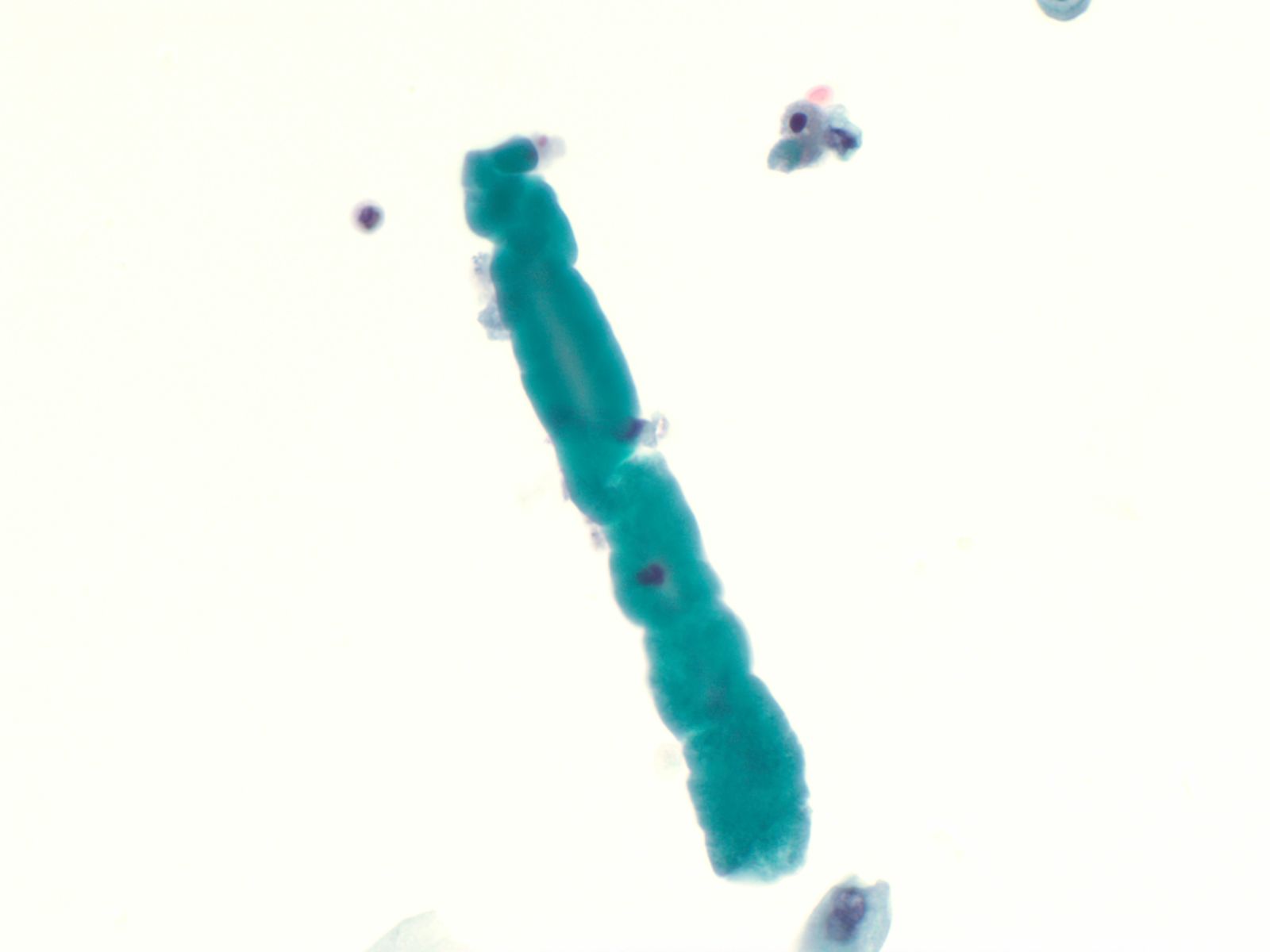
Squamous and glandular cells – N13-7020
- Squamous cells, in variable numbers, may be present as contaminants from external genitalia or may appear as a component of normal bladder being shed from the trigone
- Secretory columnar cells and cells from intestinal metaplasia may be seen as part of normal metaplastic change or as a component of cystitis glandularis
 |
 |
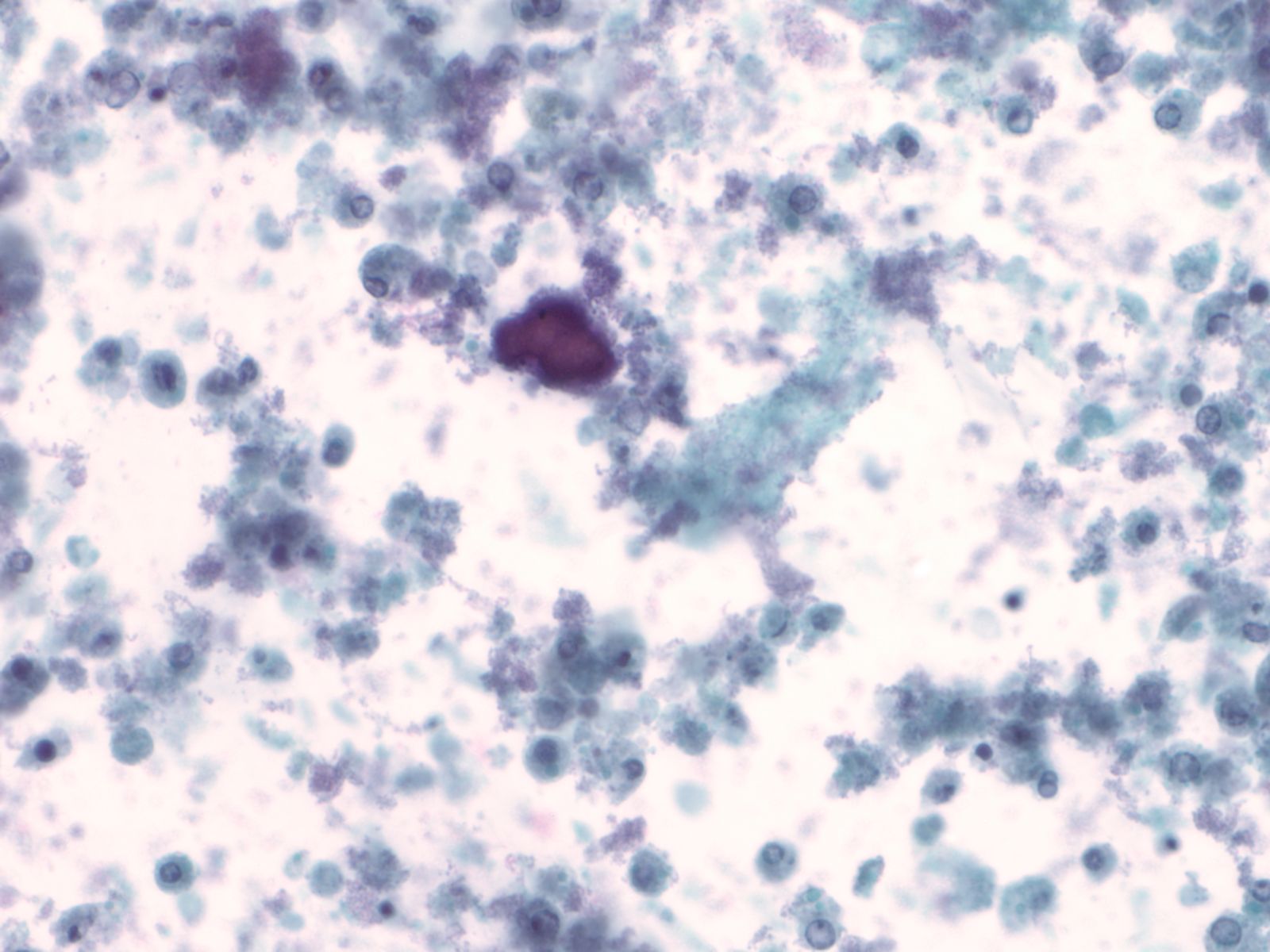
Renal casts – N13-7187
- Hyaline and granular casts may be seen even in patients without overt evidence of renal pathology
- Hyaline casts are composed of amorphous, eosinophilic proteinaceous material while the granular casts are composed of degenerated red blood cells or renal tubular cells
- Renal tubular cells are small columnar cells occurring in narrow sheets in the shape of the tubule or as single cells
 |
||
Ileal conduit urine – N13-7328
- Ileal conduit urine is usually obtained for surveillance
- Since the colonic mucosa is exposed to a hostile and toxic environment, degenerative changes predominate
- Cells resemble macrophages, there is karyorrhexis, pyknosis, and abundant red inclusions in the cytoplasm
- Cytoplasmic debris and bacteria are seen in the background
- Detection of malignancy may be challenging in this setting
- Diagnosis should be based on cells with characteristic features of malignancy
 |
||
Candida albicans – N13-7605
- Candida is the most common fungal infection and is seen as pseudo-hyphae or spores
- It may occur as a contaminant from the vagina in female patients
- However- the presence of fungal organisms in cases of renal transplant or immunosuppression denotes true infection, and requires appropriate management
Human Polyoma Virus – N13-6375
- Infection with the polyoma virus is acquired early in life
- Activation occurs for unknown reasons or in the setting of immunosuppression due to transplantation, chemotherapy, AIDS, diabetes, etc
- Infected cells vary in size but generally have an increased nuclear to cytoplasmic ratio; viral cytopathic effect is confined to the nucleus
- Inclusions of polyoma virus are large basophilic opaque and intranuclear that fill the nucleus leaving only a thin rim of residual chromatin or net-like (reticular) filaments of chromatin
- Polyoma virus changes can be mistaken for urothelial cancer and therefore these cells are called "decoy cells"; unlike carcinoma, nuclei infected with the polyoma virus tend to be round with a smooth nuclear membrane
- Polyoma virus may coexist with cancer
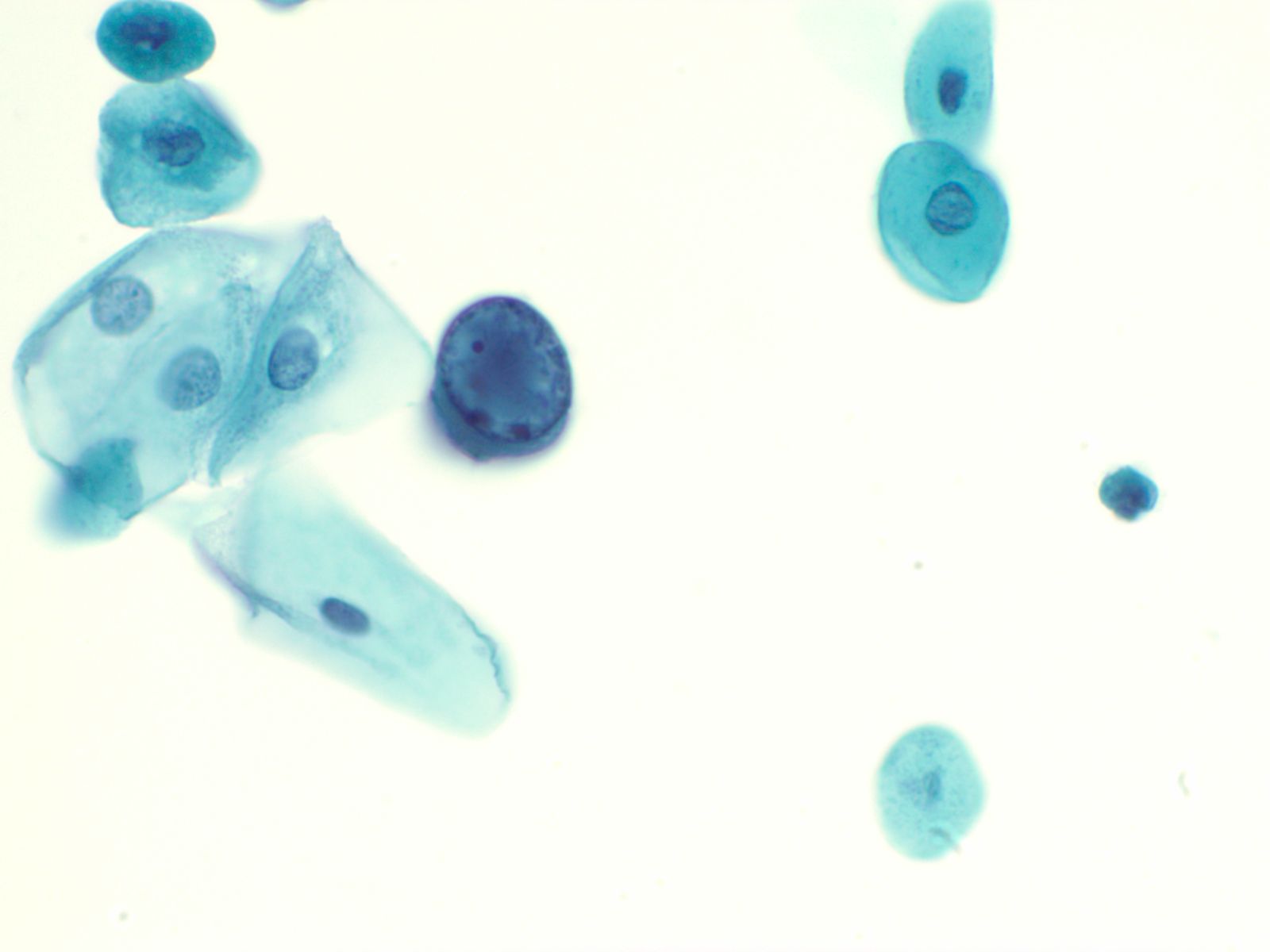 |
||
Catheterization/lithiasis changes – N13-5377
- Presence of stones can result in large urothelial clusters and papillary fragments with marked variation in the shape and size of the urothelial cells and hyperchromasia
- Sometimes the atypia associated with lithiasis is so severe that further work-up to exclude malignancy is necessary
- Cytologically, there is nuclear enlargement, pleomorphism, increased N/C ratio, coarse dense chromatin, prominent nucleoli, occasional mitoses, degeneration and necrosis
- Catheterization and instrumentation of the bladder will cause sheets and groups of urothelial cells to be sheared off; this is known as instrumentation effect and should not be confused with low grade papillary carcinoma
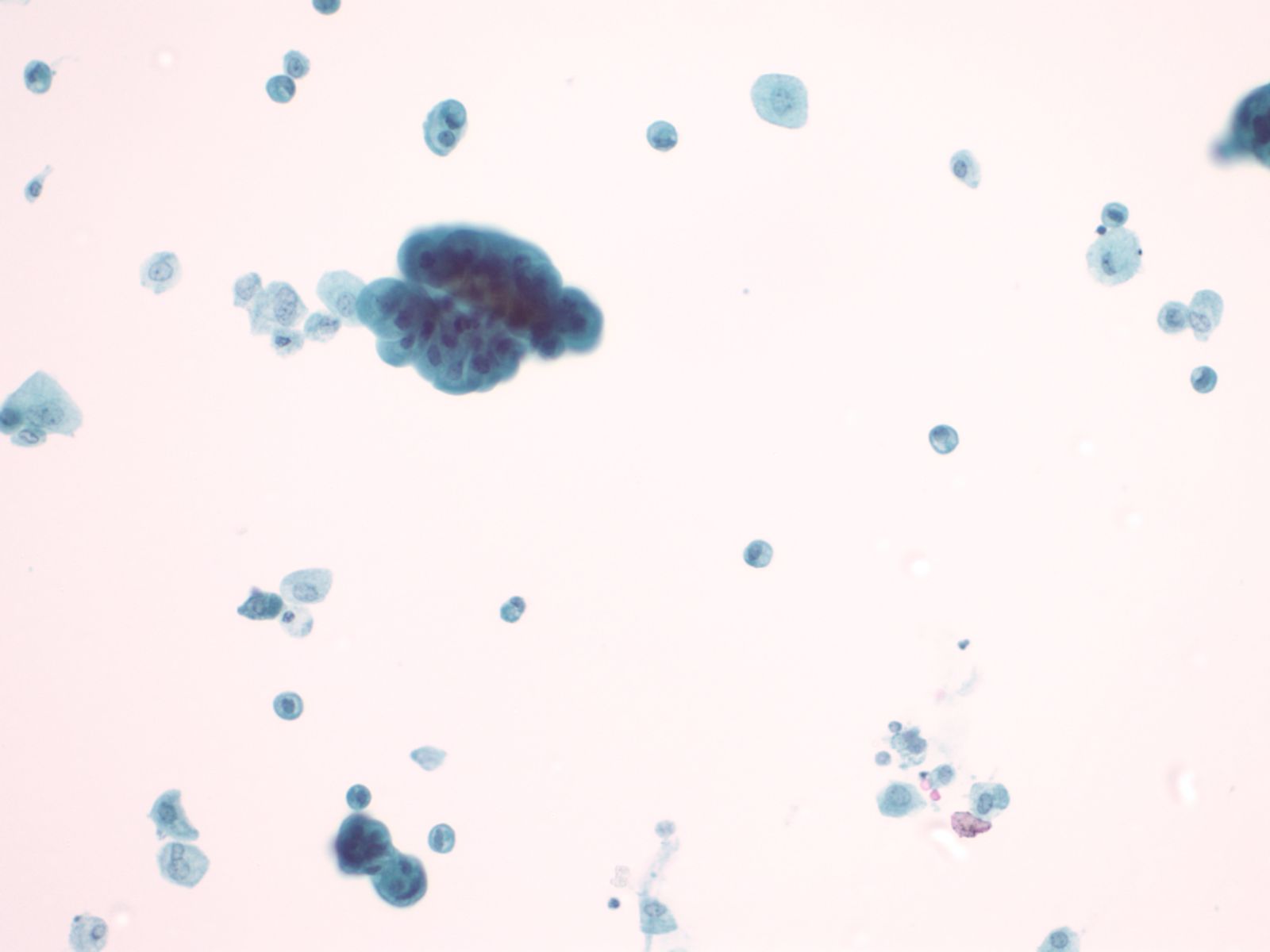 |
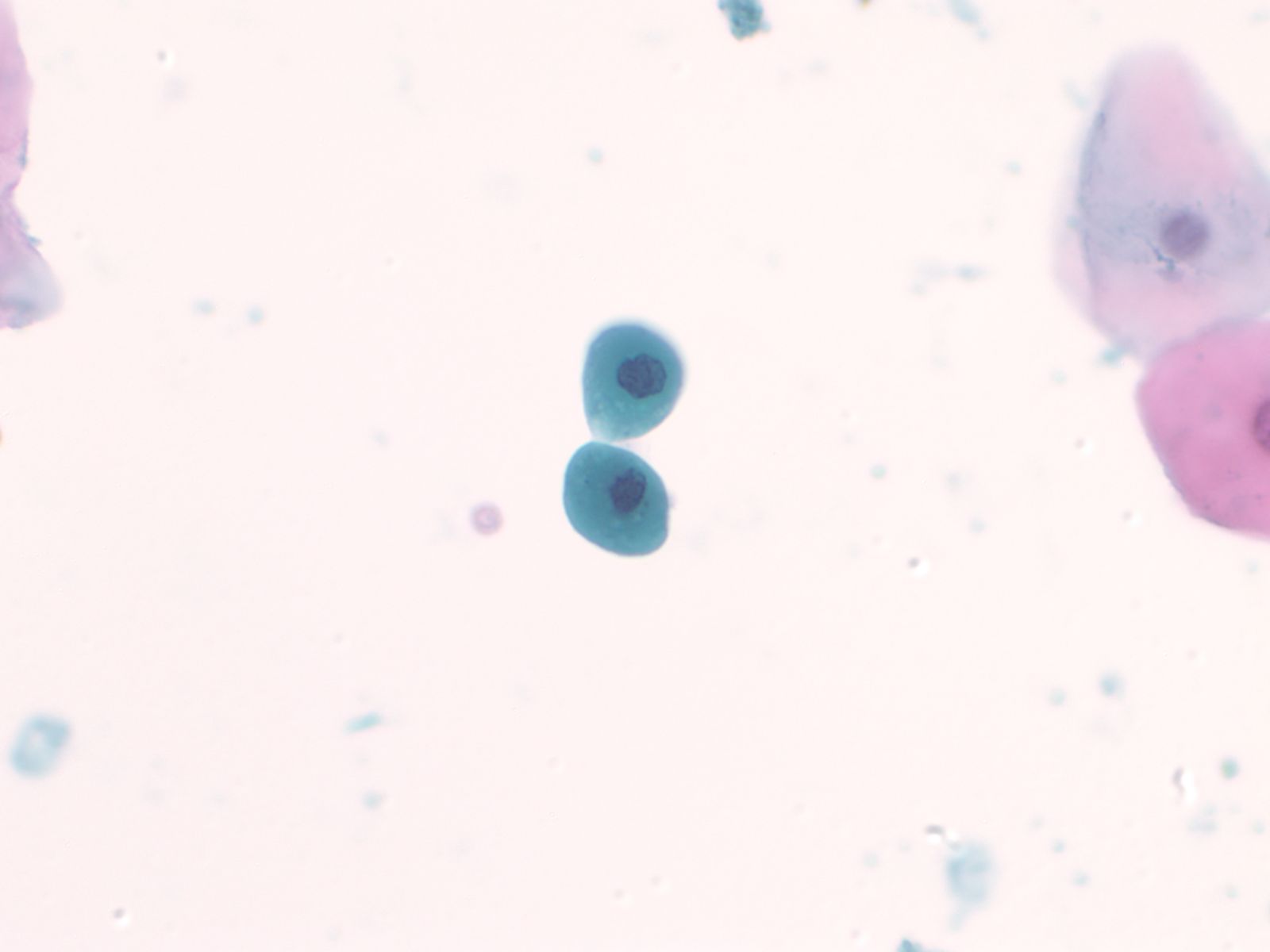 |
Urothelial carcinoma – N13-7019 and N13-6041
- Cytologically, high grade urothelial carcinomas are relatively easy to diagnose with a high degree of sensitivity and specificity due to the presence of anaplastic cells
- May see cellular preparations with abundant atypical urothelial clusters and single malignant cells in the background
- Occasionally the samples may be sparse
- Cells have high N/C ratios, with marked pleomorphism
- Nuclei are often eccentric with hyperchromatic coarse chromatin and large irregular nucleoli
- Nuclear membrane is thickened and occasional mitoses may be seen
- Cytoplasm is poorly demarcated and cyanophilic
- Overall, low grade urothelial carcinoma has a low diagnostic sensitivity and specificity because low grade tumors are diploid and lack the striking hyperchromasia of the high grade tumors.
- These tumors are cytologically bland, often impossible to distinguish from benign urothelial cells. three dimensional urothelial clusters in a voided urine, especially those with fibrovascular cores (a rare finding) are worrisome for low grade papillary TCC
- Cells in these clusters may have high N/C ratios with nuclei bulging out of the cytoplasm
- Nuclei may be irregular and may appear to have notches or grooves but more often than not the cells cannot be deemed worse than “atypical” , a diagnosis considered by urologists to be “negative”
- Chromatin is granular and evenly distributed
- Nucleoli are indistinct or absent
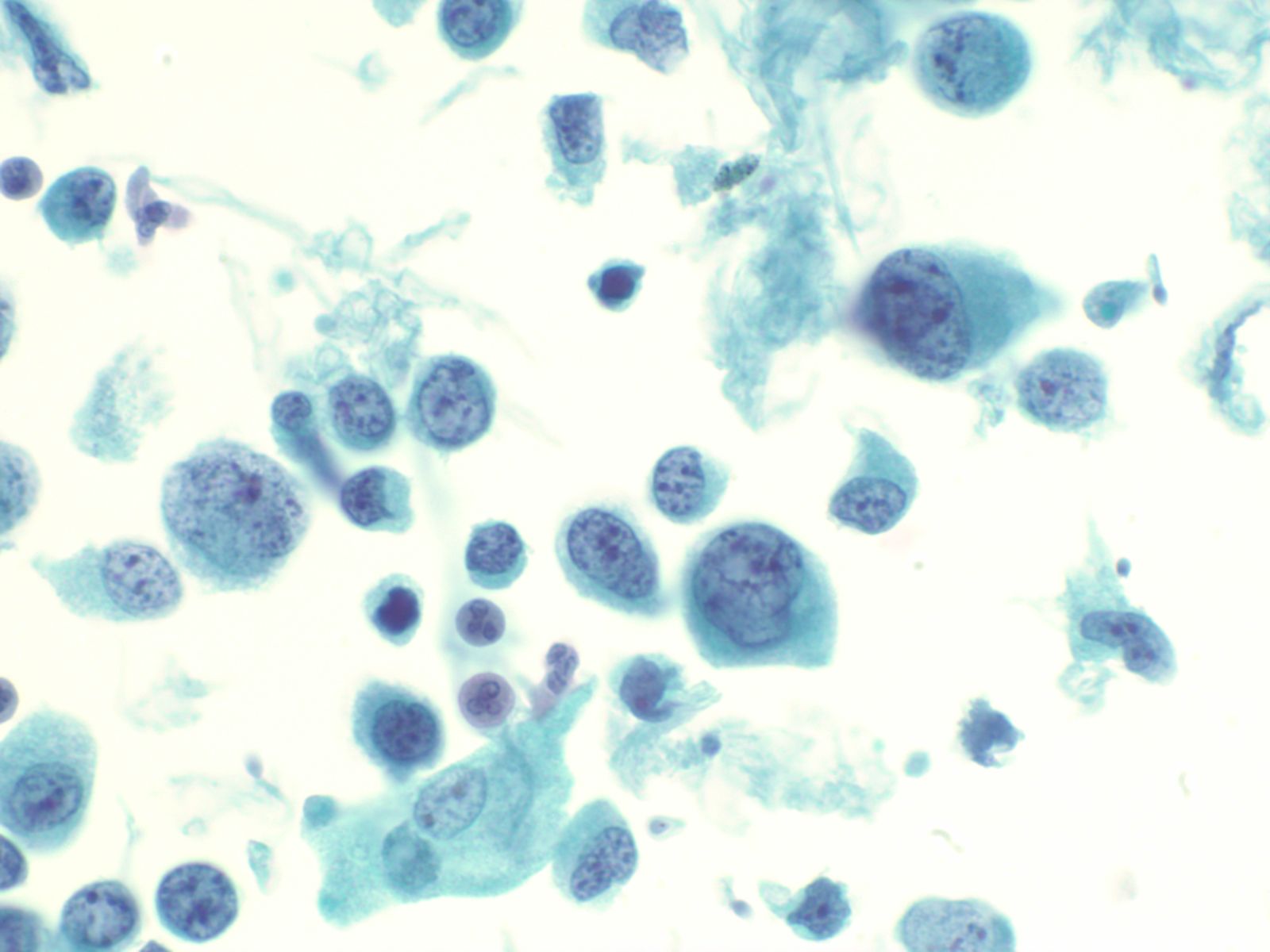 |
||
Adenocarcinoma/squamous cell carcinoma
- Primary adenocarcinomas of the bladder are rare, constituting less than 2% of all bladder cancers
- The cells have typical features of malignancy with large eccentrically placed nuclei with open chromatin and prominent nucleoli
- Cytoplasm may be abundant and may show mucin vacuoles
- Adenocarcinoma cells may be a component of a high grade urothelial carcinoma
- Primary squamous cell carcinoma of the bladder may be secondary to Schistosomiasis infection
Metastases – N13-3279
- On rare occasions, locally invasive extravesical carcinoma may invade the bladder wall and shed into the urine, such as cells of prostatic adenocarcinoma
- Distant metastases may also be found in the urine, such as carcinomas, melanomas or lymphomas
- Tumors of the female genital tract such as squamous carcinoma and adenocarcinoma of the cervix, and high grade epithelial tumors of the ovary and colon may also be occasionally seen due to direct extension of the tumors through the bladder wall
Cytology service page
Warning: Display title "1-7 Respiratory Cytology: W.S. Black-Schaffer MD, Mary Rego CT" overrides earlier display title "1-6 Urine Cytology: R. Tambouret MD, E. Brachtel MD, Ron Arpin SCT".
Lectures
- Lecture: Respiratory cytology, part 1 by Dr. WS Black-Schaffer
- Lecture: Respiratory cytology, part 2. by Dr. W.S. Black-Schaffer
- Indications for cytology examination
- Procuring the specimen
- Test platforms/specimen processing and triage
- Report
Basic cytomorphology
- Sputum
- Bronchial washing and brushings
- Bronchoalveolar lavage (BAL)
- Fine needle aspirations (FNA)
- Squamous cells (oral contamination)
- Glandular cells
- ciliated columnar
- mucous goblet cells
- Pneumocytes
- Alveolar macrophages
- Oval to bean shaped nuclei
- Mononucleated or multinucleated
- Foamy cytoplasm
- Phagocytic cells
- carbon histiocytes
- siderophages
- lipophages(associated with aspiration pneumonia)
- Seen in pt’s with TB, Sarcoid and Rheumatoid Arthritis.
- Can see giant cells
- Nodular collections of epithelioid histiocytes
- Cells with elongated nuclei with pale chromatin and tiny nucleoli
- Cartilage
- mature - homogenous, waxy
- immature - fibrillar texture
- Epithelium
- small bland bronchial cells
- Respiratory Infection –MN08-F9275 and N12-9869
- Aspergillus
- 45º angle branching of true septate
- Pneumocystis
- foamy alveolar casts
- GMS- stain cell wall of cyst black
- cysts are 4 to 8 um
- Zygomycetes-(Mucor)
- broad, irregular ribbon-like hyphae
- folding of hyphae
- Keratinizing (Well-Diff)/ Nonkeratinizing(Mod to poorly Diff)
- Bizarre cell shapes
- Cytoplasm is dense or hard with defined cell borders
- Heavy keratinization(seen in Keratinizing SCC)
- Coarsely granular and/or opaque nucleus
- Nucleoli possible
- 3-D balls, single cells, syncytia
- Lacy, fine, vacuolated cytoplasm
- Cuboidal cells, eccentric nuclei
- Finely granular irregular chromatin
- Nucleoli
- 3-D balls, papillary growth
- Finely granular to clear cytoplasm
- Eccentric nuclei
- Pale to moderately hyperchromatic chromatin
- Intranuclear cytoplasmic invaginations
- Psammoma bodies may be seen
- Single and syncytial
- Nuclear molding
- Scant cytoplasm
- Oval to angulated nuclei
- Nucleoli not prominent
- Dense chromasia (salt and pepper chromatin)
- Crush artifact ( nuclear DNA streaming)
- One to four times the size of a lymphocyte
- Uniform cuboidal cells
- Scant to moderate cytoplasm
- Small round to oval nuclei
- Stippled granular chromatin
- Small nucleoli possible
- Small blood vessels in background
- Compare the metastatic with the primary lesion
- Usually no tumor diathesis
- Presence of multiple nodules and a history of morphologically compatible cancer strongly favor metastasis
Cytology service page
Warning: Display title "1-8 Thyroid Cytology: W. Faquin MD PhD, Lisa Ring CT" overrides earlier display title "1-7 Respiratory Cytology: W.S. Black-Schaffer MD, Mary Rego CT".
Lecture slides:
by William Faquin, M.D., Ph.D.
Thyroid nodules are discovered either by palpation or by an imaging study. A palpable thyroid nodule should undergo further evaluation to determine if an FNA is warranted. Before the decision is made, a serum thyrotropin level (TSH) and thyroid ultrasound (US) should be obtained. Patients with a normal or elevated serum TSH level should proceed to a thyroid US to determine if an FNA needs to be performed. Those with a depressed serum TSH should have a radionuclide thyroid scan, the results of which should be correlated with the sonographic findings. Functioning thyroid nodules in the absence of significant clinical findings do not require an FNA because the incidence of malignancy is exceedingly low. A nodule that appears either iso- or hypo-functioning on radionuclide scan should be considered for FNA based on the US findings.
Incidental thyroid nodules (“incidentalomas”) are detected by US, FDG-PET, sestamibi, CT, and MRI scans. Those detected by US have a cancer risk of approximately 10-15% (0-29%) and should undergo dedicated thyroid sonographic evaluation. Lesions with a maximum diameter greater than 1.0-1.5 cm should be considered for biopsy unless they are simple cysts or septated cysts with no solid elements. FNA may also occasionally be replaced by periodic follow-up for nodules of borderline size (between 1.0-1.5cm in maximum diameter) if they have sonographic features that are strongly associated with benign cytology.
A nodule of any size with sonographically suspicious features should also be considered for FNA. Sonographically suspicious features include microcalcifications, hypoechoic solid nodules, irregular/lobulated margins, intra-nodular vascularity, and nodal metastases (or signs of extracapsular spread). This recommendation is controversial because it includes patients with microcarcinomas, in whom a survival benefit following an FNA diagnosis has not been documented. If a sonographically suspicious nodule is benign by FNA, the patient can be reassured, and subsequent follow-up can be less frequent. On the other hand, if the FNA reveals that the nodule is malignant, surgery is generally recommended. The natural history of papillary microcarcinomas, however, is not well understood. Most remain indolent, as implied by the 13% prevalence of papillary microcarcinomas in the United States at autopsy examination. A minority follow a more aggressive course; this subgroup might be identified by sonographic evidence of lateral cervical node metastases, tumor multifocality, extrathyroidal invasion, or cytopathologic features that suggest a high-grade malignancy.
by William Faquin, M.D., Ph.D.
The fine-needle aspiration biopsy (FNA) was introduced for the first time in the U.S.A. in the 1930s but was only during the 1950s in Sweden that it was widely appreciated as a diagnostic tool. Since then this method has spread worldwide because of its simplicity, safety and the possibility of repetition.
The incidence and mortality of thyroid cancer do not qualify it as an important public health problem but the number of surgical lobectomies done to establish or to exclude its presence makes it a disease of economic importance. In this setting FNA is regarded as the most accurate method for the selection of patients with thyroid nodules and a very cost-effective diagnostic test.
The FNA of a thyroid nodule is preferably carried out under sonographic guidance, although when easily palpable, the maneuver can be performed under manual guidance. All the nodules in a multinodular goiter should be aspirated because the risk of malignancy is the same in each nodule but usually they are selected by the sonographer based on their ultrasound (US) appearance. A hypoechoic solid pattern with irregular margins and the presence of intralesional calcium deposits are the most important clues for suspecting a malignant lesion. Another useful method of nodule selection is the evaluation of its Echo-Power Doppler pattern: if a nodule is vascularized the likelihood of malignancy is higher compared to poorly vascularized lesions. The aspiration is performed with thin needles (gauge from 27G to 20G) and it is important to note that the amount of cells does not depend on the caliber of the needle but on the sampling time. Therefore, thyroid lesions which are usually richly vascularized are better sampled using very thin needles (either 27 or 25G) rather than larger ones (23 to 20G). After applying superficial anesthesia, which may be carried out by spraying the skin with ethyl chloride or by injecting lidocaine into the subcutis, the operator holds the sonographic probe with one hand and performs the aspiration with the other by means of a syringe-holding pistol. A FNA may also be carried out by simply moving the needle, without any connection to a suction device (cytopuncture): in this case the material is extruded from the lesion by capillarity. The risk of complications is low (see below) even when the number of FNA passes is up to 5 for each nodule. The procedure can be repeated safely when the smear shows low cellularity at the on-site assessment and a reliable diagnosis cannot be rendered. When on-site assessment of specimen adequacy is available, 2 passes are usually sufficient. However, when on-site evaluation is not possible or when liquid-based cytology is chosen 3-5 passes might be required depending on the skill of the operator and on the characteristics of the lesion.
There is no agreement in the literature as to whether the pathologist should or should not perform the FNA on his own. If the pathologist performs the aspiration, an immediate evaluation of the sample adequacy is possible. Clinicians are generally more familiar with the clinical aspects of the case; however, the different non-diagnostic rates by clinicians suggest that experience is an essential requisite to obtain an adequate cytological sample. Regardless of the subspecialty of the operator, the procedure should be carried out with appropriate frequency (at least 100 F.N.A.B./ year) to maintain competency.
by William Faquin, M.D., Ph.D.
When possible, the salivary gland FNA should be performed by, or in collaboration with, a cytopathologist in order that a preliminary interpretation of the sample can be made before the procedure is completed. This allows for assessment of sample adequacy, but it also permits the triage of the sample for ancillary studies. For example, the needle rinsings from lymphoid lesions can be sent for flow cytometric evaluation for potential lymphoproliferative lesions, a cell block can be made for cases where histochemical and immunohistochemical studies will be needed, microbiologic cultures can be sent for lesions that appear to be inflammatory/infectious, and a sample can be placed into glutaraldehyde fixative for ultrastructural studies on selected challenging cases.
A combination of both alcohol-fixed and air-dried smears are essential to maximize the diagnostic evaluation of a salivary gland FNA sample. Because many salivary gland tumors contain a variable combination of cells and matrix material, Diff-Quik and Papanicoloau stains are complimentary in the evaluation of salivary gland aspirates Diff-Quik staining highlights the cytologic features and tinctorial properties of any matrix material that may be present. This is key in distinguishing certain common salivary gland tumors such as pleomorphic adenoma and adenoid cystic carcinoma (see Chapter 6) where the appearance of the matrix rather than the cells is the most important diagnostic feature (Fig. 2.4). Diff-Quik stained smears are also more useful than Papanicoloau-stained preparations for the evaluation of cytoplasmic vacuoles as in acinic cell carcinomas (see Chapter 8). In addition, for the rapid assessment of an FNA specimen, air-dried Diff-Quik preparations are much less time-consuming to prepare. Alcohol-fixation and Papanicoloau staining is useful for better visualizing the nuclear features of the cell including chromatin pattern, nuclear membrane irregulaties, nucleoli, and inclusions. In our opinion, a detailed evaluation of nuclear atypia is best achieved using Papanicoloau staining.
As an adjunct to standard smears, needle rinsings are important since they can be used to produce a thin-layer preparation, cytospin and/or cellblock. Thin-layer (TP) or cytospin preparations in our opinion should not be the sole method for evaluating salivary gland lesions, especially those containing matrix material. TPs do have the advantage of concentrating the cells onto a single slide and of removing excess obscuring blood. For cystic lesions where the cellular components are diluted within a large volume, TP is probably the best preparatory method to use. A cellblock can be prepared from the needle rinsings for those cases where histochemical (e.g. mucicarmine, PTAH) or immunohistochemical (e.g. S-100, cytokeratin) stains would be useful in the diagnostic evaluation.
by William Faquin, M.D., Ph.D.
Salivary gland cytopathology is a diagnostically challenging area in part because of the wide variety of neoplasms arising in the salivary glands and the overlapping cytomorphologic features of so many of these tumors. As they say, “forewarned is forearmed;” by being aware of certain specific problem areas in salivary gland cytology, one can more readily avoid diagnostic errors. Within the context of the algorithm presented in Chapter 3 and applied within subsequent chapters of this book, we will address specific problem areas in detail. Below is a list of some of the most common problem areas of salivary gland FNA where the cytologist should be particularly cautious. Standard reporting terminology is used including: Non-diagnostic, Negative for malignant cells, Atypical, Suspicious for malignancy, and Malignant.
Diagnostically Challenging Areas of Salivary Gland Cytology: Matrix-containing lesions: pleomorphic adenoma versus adenoid cystic carcinoma Basaloid neoplasms: basal cell adenoma and basal cell adenocarcinoma versus solid variant of adenoid cystic carcinoma Oncocytic lesions: Warthin tumor and oncocytoma versus acinic cell carcinoma Mucinous cysts: low-grade mucoepidermoid carcinoma versus mucocele High-grade carcinomas: high grade mucoepidermoid carcinoma versus salivary duct carcinoma and metastasis Lymphoid lesions: lymphoepithelial sialadenitis (LESA) vs lymphoma Clear cell tumors: epithelial-myoepithelial carcinoma vs myoepithelioma Spindle cell lesions: schwannoma vs myoepithelioma
Basic cytomorphology
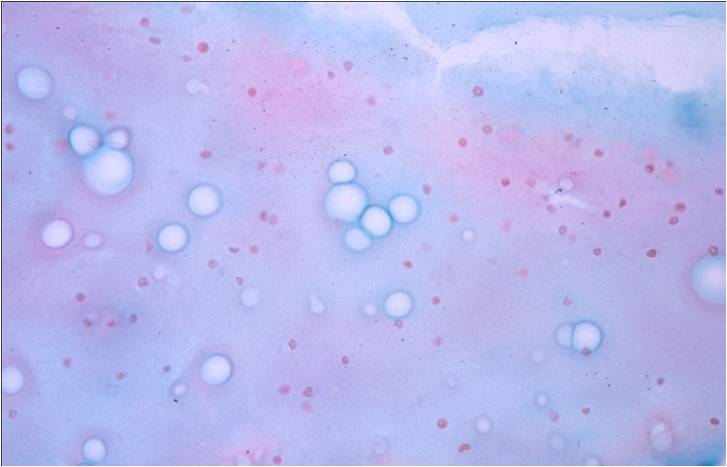
Benign Thyroid –THY4-16
- Adequacy: must meet a minimum of 6 groups of well-visualized follicular cells with at least 10 cells per group
- Abundant watery colloid in background
- Scattered fragments of macrofollicles
- Normal follicular cells contain small amounts of granular cytoplasm with a small, dark round central nucleus
- May find few macrophages (foam cells)
- Reactive Hurthle cell changes can be seen
 |
 |
 |
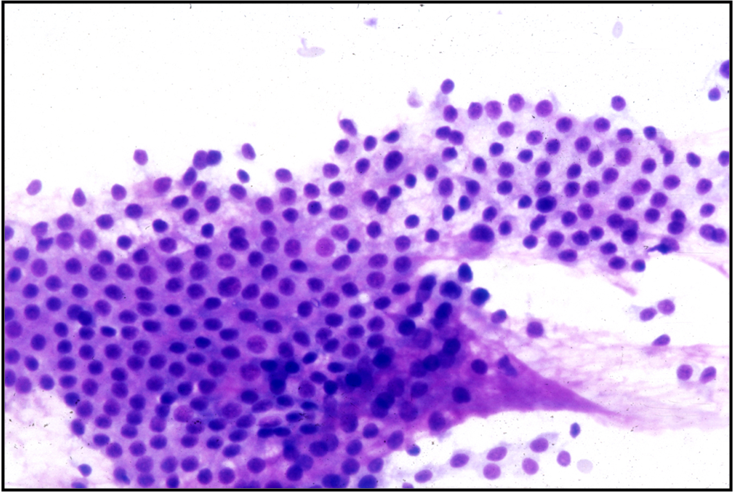 |
Hashimoto Thyroiditis (chronic lymphocytic thyroiditis) –THY2-15
- 2 cell types NEEDED for diagnosis: lymphocytes and Hürthle cells!
- Lymphocytes embedded within the groups of follicular/Hürthle cells are a characteristic feature
- Hürthle cells may show increased N/C ratio, prominent nucleoli and nuclear irregularity
- Small amounts of dense colloid may be present
- Squamous metaplastic cells, foam cells, giant cells, fibrous tissue, granulomas and calcifications may be present
- Tingible body macrophages and germinal center fragments
- Plasma cells
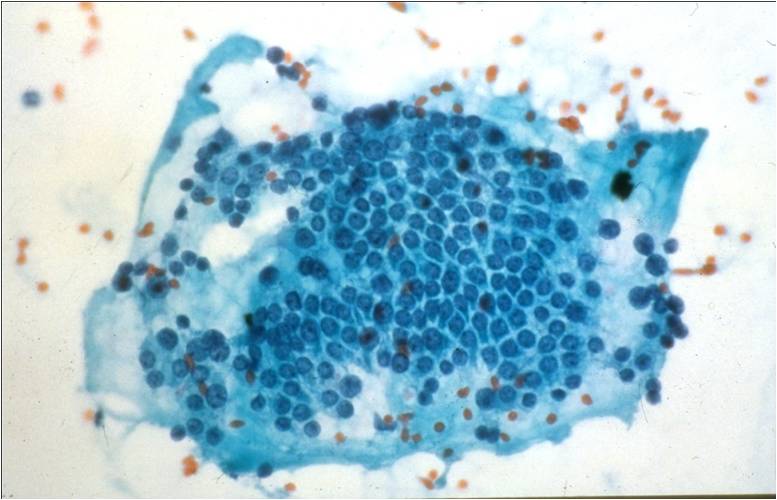
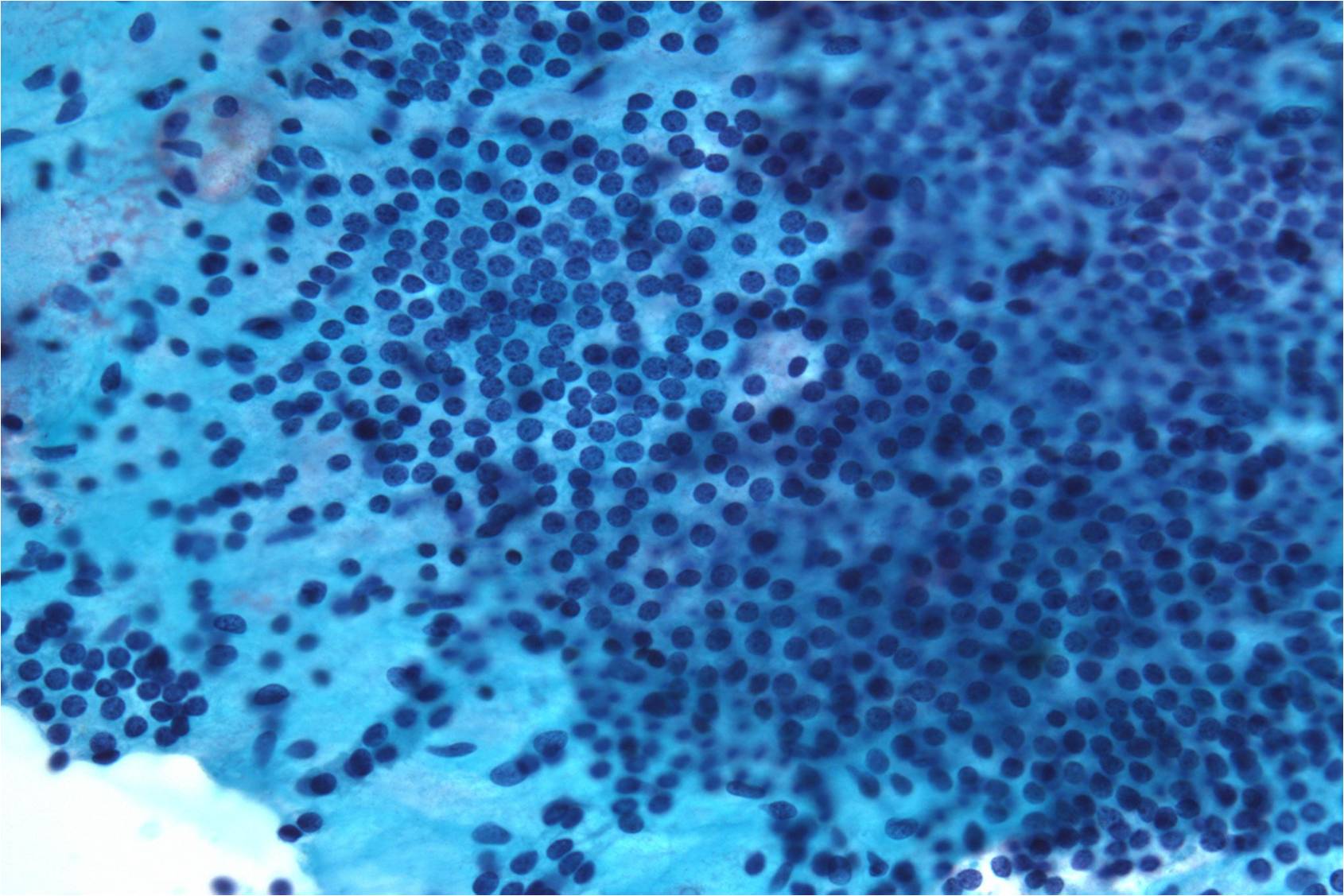
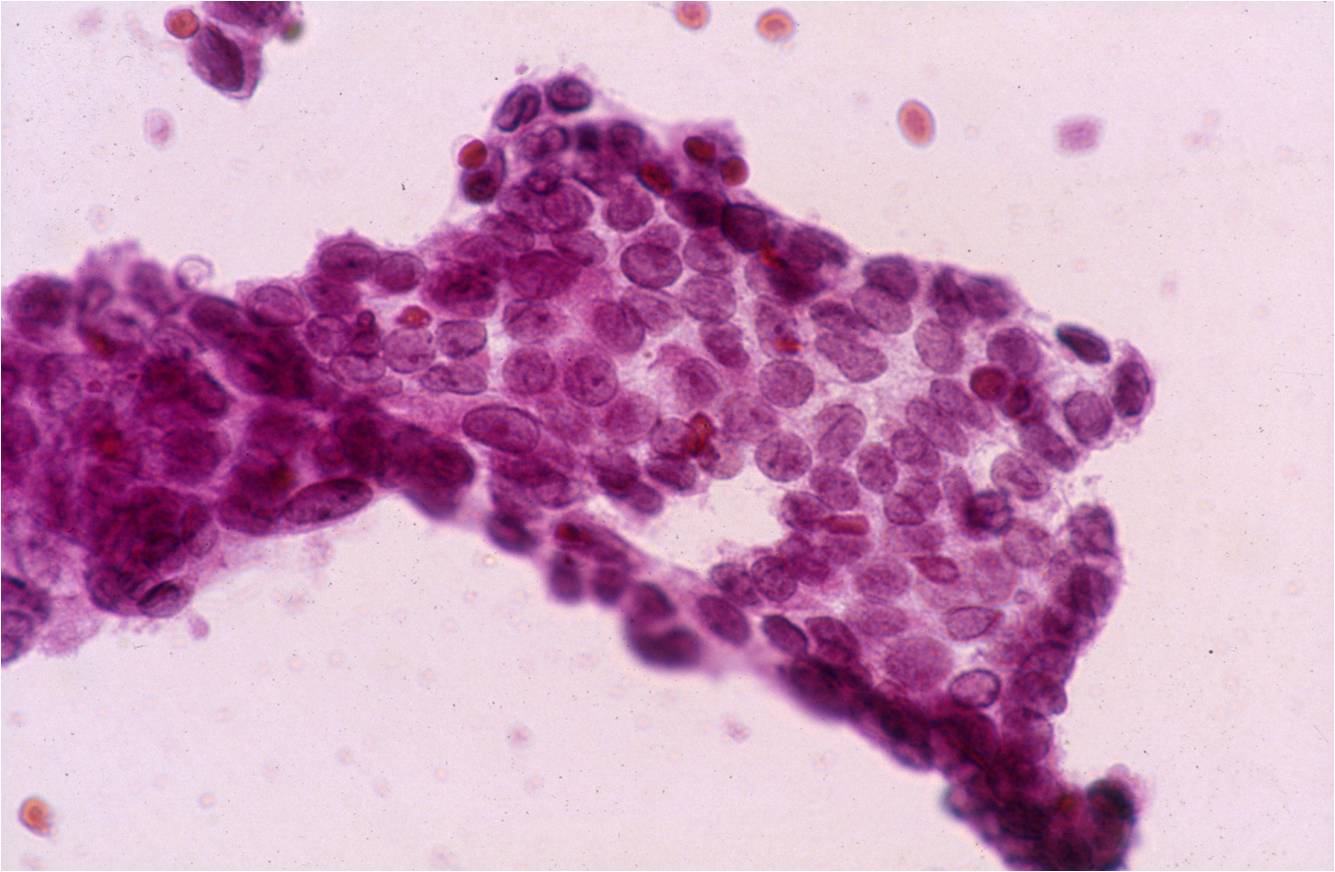
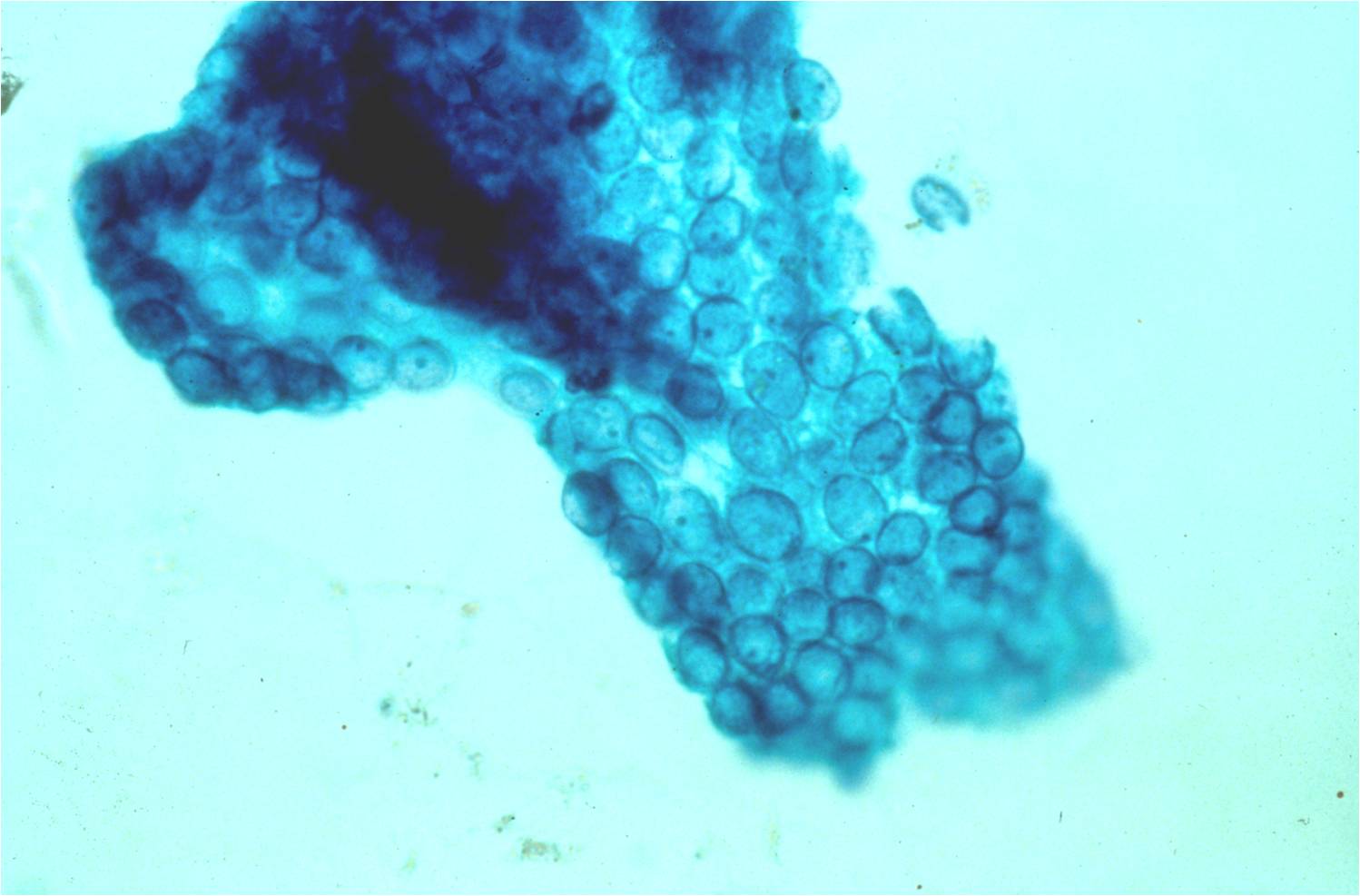
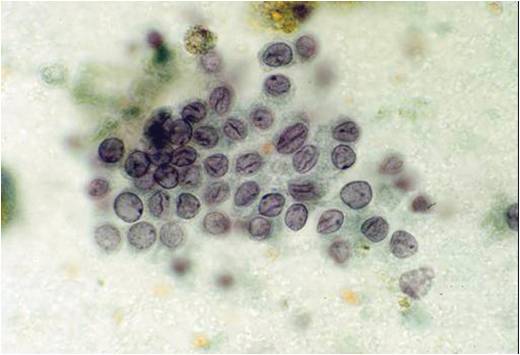
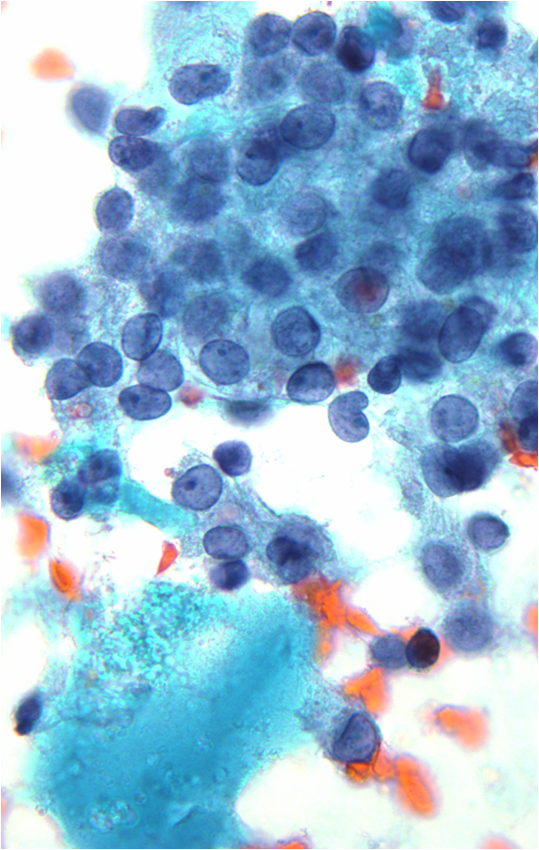
Papillary Carcinoma –THY2-26
- Increased cellularity!
- 3D papillary architecture or monolayered sheets
- Intranuclear inclusions
- Nuclear grooves (less specific for diagnosis)
- Pale even chromatin
- May see small but prominent nucleoli
- Variation in nuclear size and shapes
- Generally abundant cytoplasm that is either dense and homogenous or granular but well defined borders
- Blood in background
- SCANT colloid (or very little--dense, bubble-gum appearance)
- Psammoma bodies
 |
 |
 |
 |
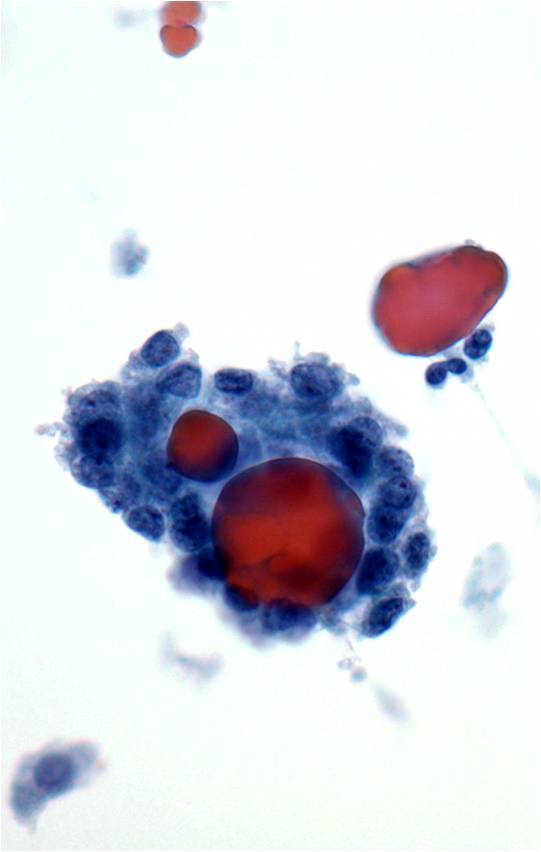 |
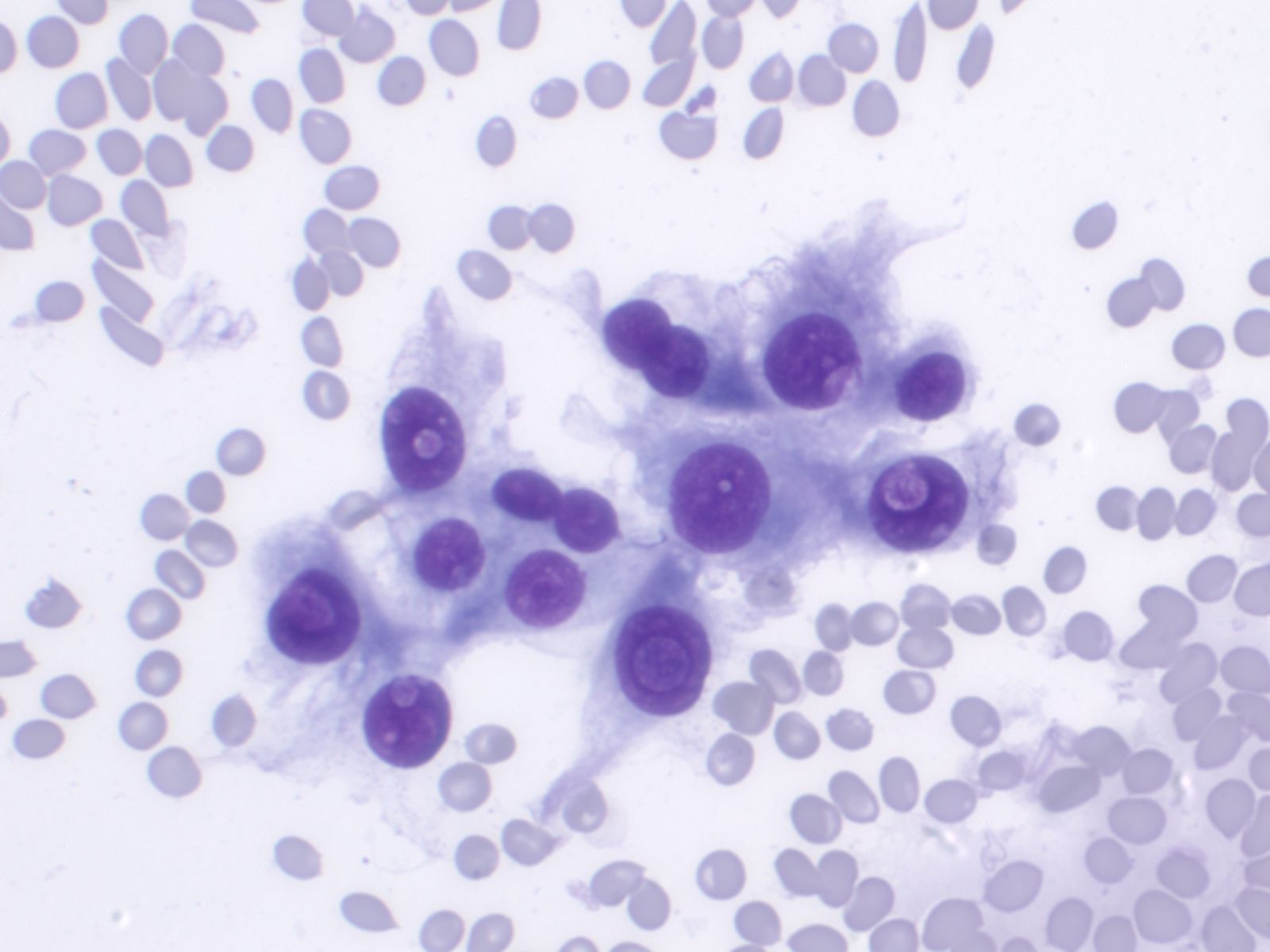 |
Medullary Carcinoma –THY4-05
- Malignant parafollicular C-cells
- CELLULAR SMEAR with isolated cells and some loosely cohesive cell clusters
- Mild to absent pleomorphism
- Can have a neuroendocrine-like appearance--salt & pepper chromatin pattern
- Intranuclear pseudoinclusions are also present
- Cytoplasm is finely granular and may contain red granules (seen in 30% of cases with Diff-Quik)
- Cell types include plasmacytoid, spindle, polygonal, round or triangular
- Amyloid--looks like dense colloid (stains bright green with Congo Red)
Undifferentiated (Anaplastic) Carcinoma –THY5-19
- Increased cellularity
- Malignant features
- Epithelioid cells present in groups and singly--round, spindled or polygonal in shape
- Pleomorphic nuclei--may see bi or multinucleated cells
- Increased N/C ratios
- Cytoplasm is moderate to abundant, basophilic and well-defined which sometimes may be vacuolated or granular
- Prominent nucleoli
- Irregular, clumped, coarse chromatin patterns
- May find intranuclear inclusions
- May see mitoses
- Background is usually rich in necrotic debris and blood
Cytology service page
Warning: Display title "1-9 Salivary Gland and Head and Neck Cases: W.C. Faquin MD PhD, Lisa Ring CT" overrides earlier display title "1-8 Thyroid Cytology: W. Faquin MD PhD, Lisa Ring CT".
General
by William Faquin, M.D., Ph.D.
Thyroid nodules are discovered either by palpation or by an imaging study. A palpable thyroid nodule should undergo further evaluation to determine if an FNA is warranted. Before the decision is made, a serum thyrotropin level (TSH) and thyroid ultrasound (US) should be obtained. Patients with a normal or elevated serum TSH level should proceed to a thyroid US to determine if an FNA needs to be performed. Those with a depressed serum TSH should have a radionuclide thyroid scan, the results of which should be correlated with the sonographic findings. Functioning thyroid nodules in the absence of significant clinical findings do not require an FNA because the incidence of malignancy is exceedingly low. A nodule that appears either iso- or hypo-functioning on radionuclide scan should be considered for FNA based on the US findings.
Incidental thyroid nodules (“incidentalomas”) are detected by US, FDG-PET, sestamibi, CT, and MRI scans. Those detected by US have a cancer risk of approximately 10-15% (0-29%) and should undergo dedicated thyroid sonographic evaluation. Lesions with a maximum diameter greater than 1.0-1.5 cm should be considered for biopsy unless they are simple cysts or septated cysts with no solid elements. FNA may also occasionally be replaced by periodic follow-up for nodules of borderline size (between 1.0-1.5cm in maximum diameter) if they have sonographic features that are strongly associated with benign cytology.
A nodule of any size with sonographically suspicious features should also be considered for FNA. Sonographically suspicious features include microcalcifications, hypoechoic solid nodules, irregular/lobulated margins, intra-nodular vascularity, and nodal metastases (or signs of extracapsular spread). This recommendation is controversial because it includes patients with microcarcinomas, in whom a survival benefit following an FNA diagnosis has not been documented. If a sonographically suspicious nodule is benign by FNA, the patient can be reassured, and subsequent follow-up can be less frequent. On the other hand, if the FNA reveals that the nodule is malignant, surgery is generally recommended. The natural history of papillary microcarcinomas, however, is not well understood. Most remain indolent, as implied by the 13% prevalence of papillary microcarcinomas in the United States at autopsy examination. A minority follow a more aggressive course; this subgroup might be identified by sonographic evidence of lateral cervical node metastases, tumor multifocality, extrathyroidal invasion, or cytopathologic features that suggest a high-grade malignancy.
by William Faquin, M.D., Ph.D.
The fine-needle aspiration biopsy (FNA) was introduced for the first time in the U.S.A. in the 1930s but was only during the 1950s in Sweden that it was widely appreciated as a diagnostic tool. Since then this method has spread worldwide because of its simplicity, safety and the possibility of repetition.
The incidence and mortality of thyroid cancer do not qualify it as an important public health problem but the number of surgical lobectomies done to establish or to exclude its presence makes it a disease of economic importance. In this setting FNA is regarded as the most accurate method for the selection of patients with thyroid nodules and a very cost-effective diagnostic test.
The FNA of a thyroid nodule is preferably carried out under sonographic guidance, although when easily palpable, the maneuver can be performed under manual guidance. All the nodules in a multinodular goiter should be aspirated because the risk of malignancy is the same in each nodule but usually they are selected by the sonographer based on their ultrasound (US) appearance. A hypoechoic solid pattern with irregular margins and the presence of intralesional calcium deposits are the most important clues for suspecting a malignant lesion. Another useful method of nodule selection is the evaluation of its Echo-Power Doppler pattern: if a nodule is vascularized the likelihood of malignancy is higher compared to poorly vascularized lesions. The aspiration is performed with thin needles (gauge from 27G to 20G) and it is important to note that the amount of cells does not depend on the caliber of the needle but on the sampling time. Therefore, thyroid lesions which are usually richly vascularized are better sampled using very thin needles (either 27 or 25G) rather than larger ones (23 to 20G). After applying superficial anesthesia, which may be carried out by spraying the skin with ethyl chloride or by injecting lidocaine into the subcutis, the operator holds the sonographic probe with one hand and performs the aspiration with the other by means of a syringe-holding pistol. A FNA may also be carried out by simply moving the needle, without any connection to a suction device (cytopuncture): in this case the material is extruded from the lesion by capillarity. The risk of complications is low (see below) even when the number of FNA passes is up to 5 for each nodule. The procedure can be repeated safely when the smear shows low cellularity at the on-site assessment and a reliable diagnosis cannot be rendered. When on-site assessment of specimen adequacy is available, 2 passes are usually sufficient. However, when on-site evaluation is not possible or when liquid-based cytology is chosen 3-5 passes might be required depending on the skill of the operator and on the characteristics of the lesion.
There is no agreement in the literature as to whether the pathologist should or should not perform the FNA on his own. If the pathologist performs the aspiration, an immediate evaluation of the sample adequacy is possible. Clinicians are generally more familiar with the clinical aspects of the case; however, the different non-diagnostic rates by clinicians suggest that experience is an essential requisite to obtain an adequate cytological sample. Regardless of the subspecialty of the operator, the procedure should be carried out with appropriate frequency (at least 100 F.N.A.B./ year) to maintain competency.
by William Faquin, M.D., Ph.D.
When possible, the salivary gland FNA should be performed by, or in collaboration with, a cytopathologist in order that a preliminary interpretation of the sample can be made before the procedure is completed. This allows for assessment of sample adequacy, but it also permits the triage of the sample for ancillary studies. For example, the needle rinsings from lymphoid lesions can be sent for flow cytometric evaluation for potential lymphoproliferative lesions, a cell block can be made for cases where histochemical and immunohistochemical studies will be needed, microbiologic cultures can be sent for lesions that appear to be inflammatory/infectious, and a sample can be placed into glutaraldehyde fixative for ultrastructural studies on selected challenging cases.
A combination of both alcohol-fixed and air-dried smears are essential to maximize the diagnostic evaluation of a salivary gland FNA sample. Because many salivary gland tumors contain a variable combination of cells and matrix material, Diff-Quik and Papanicoloau stains are complimentary in the evaluation of salivary gland aspirates Diff-Quik staining highlights the cytologic features and tinctorial properties of any matrix material that may be present. This is key in distinguishing certain common salivary gland tumors such as pleomorphic adenoma and adenoid cystic carcinoma (see Chapter 6) where the appearance of the matrix rather than the cells is the most important diagnostic feature (Fig. 2.4). Diff-Quik stained smears are also more useful than Papanicoloau-stained preparations for the evaluation of cytoplasmic vacuoles as in acinic cell carcinomas (see Chapter 8). In addition, for the rapid assessment of an FNA specimen, air-dried Diff-Quik preparations are much less time-consuming to prepare. Alcohol-fixation and Papanicoloau staining is useful for better visualizing the nuclear features of the cell including chromatin pattern, nuclear membrane irregulaties, nucleoli, and inclusions. In our opinion, a detailed evaluation of nuclear atypia is best achieved using Papanicoloau staining.
As an adjunct to standard smears, needle rinsings are important since they can be used to produce a thin-layer preparation, cytospin and/or cellblock. Thin-layer (TP) or cytospin preparations in our opinion should not be the sole method for evaluating salivary gland lesions, especially those containing matrix material. TPs do have the advantage of concentrating the cells onto a single slide and of removing excess obscuring blood. For cystic lesions where the cellular components are diluted within a large volume, TP is probably the best preparatory method to use. A cellblock can be prepared from the needle rinsings for those cases where histochemical (e.g. mucicarmine, PTAH) or immunohistochemical (e.g. S-100, cytokeratin) stains would be useful in the diagnostic evaluation.
by William Faquin, M.D., Ph.D.
Salivary gland cytopathology is a diagnostically challenging area in part because of the wide variety of neoplasms arising in the salivary glands and the overlapping cytomorphologic features of so many of these tumors. As they say, “forewarned is forearmed;” by being aware of certain specific problem areas in salivary gland cytology, one can more readily avoid diagnostic errors. Within the context of the algorithm presented in Chapter 3 and applied within subsequent chapters of this book, we will address specific problem areas in detail. Below is a list of some of the most common problem areas of salivary gland FNA where the cytologist should be particularly cautious. Standard reporting terminology is used including: Non-diagnostic, Negative for malignant cells, Atypical, Suspicious for malignancy, and Malignant.
Diagnostically Challenging Areas of Salivary Gland Cytology: Matrix-containing lesions: pleomorphic adenoma versus adenoid cystic carcinoma Basaloid neoplasms: basal cell adenoma and basal cell adenocarcinoma versus solid variant of adenoid cystic carcinoma Oncocytic lesions: Warthin tumor and oncocytoma versus acinic cell carcinoma Mucinous cysts: low-grade mucoepidermoid carcinoma versus mucocele High-grade carcinomas: high grade mucoepidermoid carcinoma versus salivary duct carcinoma and metastasis Lymphoid lesions: lymphoepithelial sialadenitis (LESA) vs lymphoma Clear cell tumors: epithelial-myoepithelial carcinoma vs myoepithelioma Spindle cell lesions: schwannoma vs myoepithelioma
Basic cytomorphology
Benign Salivary Gland Tissue – SG2-05
- Acinar structures separated by adipose tissue
- Serous acinar cell cytoplasm contains zymogen granules (basophilic on Diff-Quik): produce enzymes
- Mucinous cells are tall, columnar with abundant, finely granular or vacuolated cytoplasm and basally located small round nuclei
- Ductal epithelium has scant cytoplasm and round to oval small, dark nuclei
- Myoepithelial cells are rarely seen as an isolated component in normal tissue and they are closely associated with epithelial structures
- Myoepithelial cells have oval to plasmacytoid shape, oval to round nuclei, moderate amounts of cytoplasm or as bare nuclei
- Other variable cell types: oncocytes (increase with age), metaplastic squamous cells, sebaceous cells, lymphoid cells
Pleomorphic Adenoma –SG2-22
- Most common benign tumor of parotid gland (75%), well-circumscribed mass
- Aspirates have a thick, gelatinous consistency 3 components NEEDED for diagnosis: epithelial cells, myoepithelial cells and matrix
- Matrix- fibrillar with ragged edges and embedded myoepithelial cells
- Dusty purple stain on pap stain: magenta using air dried preps
- Slight atypia in stroma may signify degeneration
- Epithelium arranged in loosely cohesive groups, flat sheets and glands
- Admixture of cellular and stromal components shows a characteristic blending, not seen in other salivary gland tumors
- Myoepithelial cells may be associated with epithelial groups or may appear as "naked" nuclei in the background of smears
- May see metaplastic squamous cells or oncocytes surrounding tumor
 |
 |
|
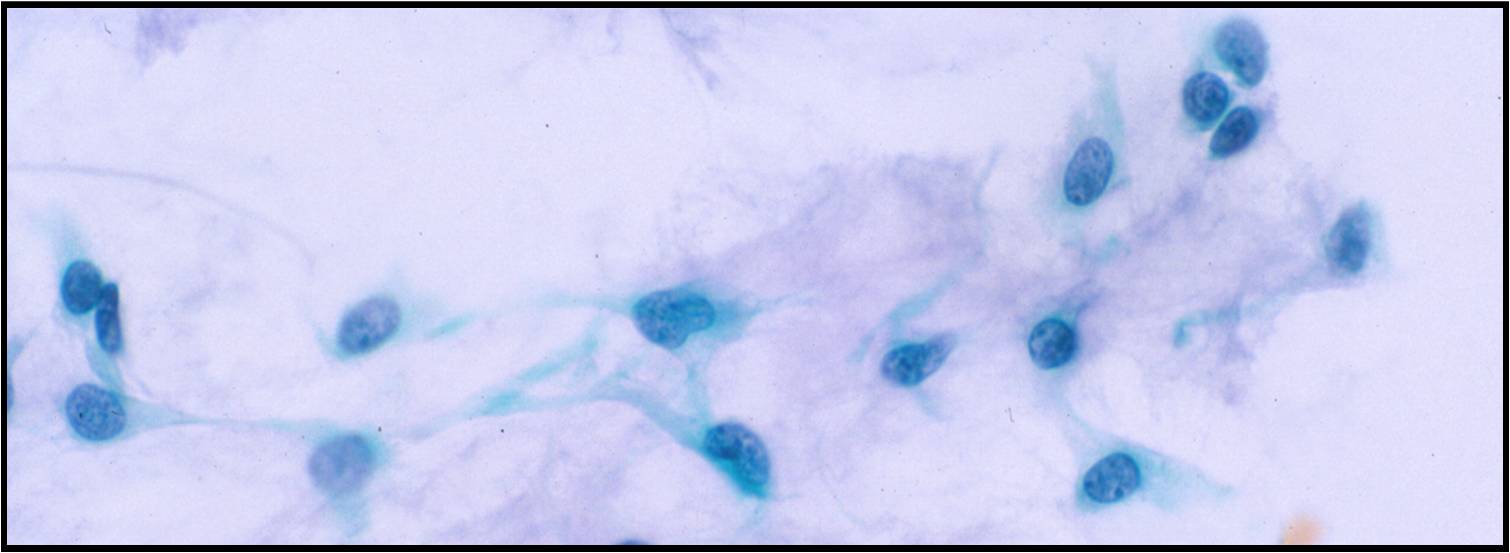 |
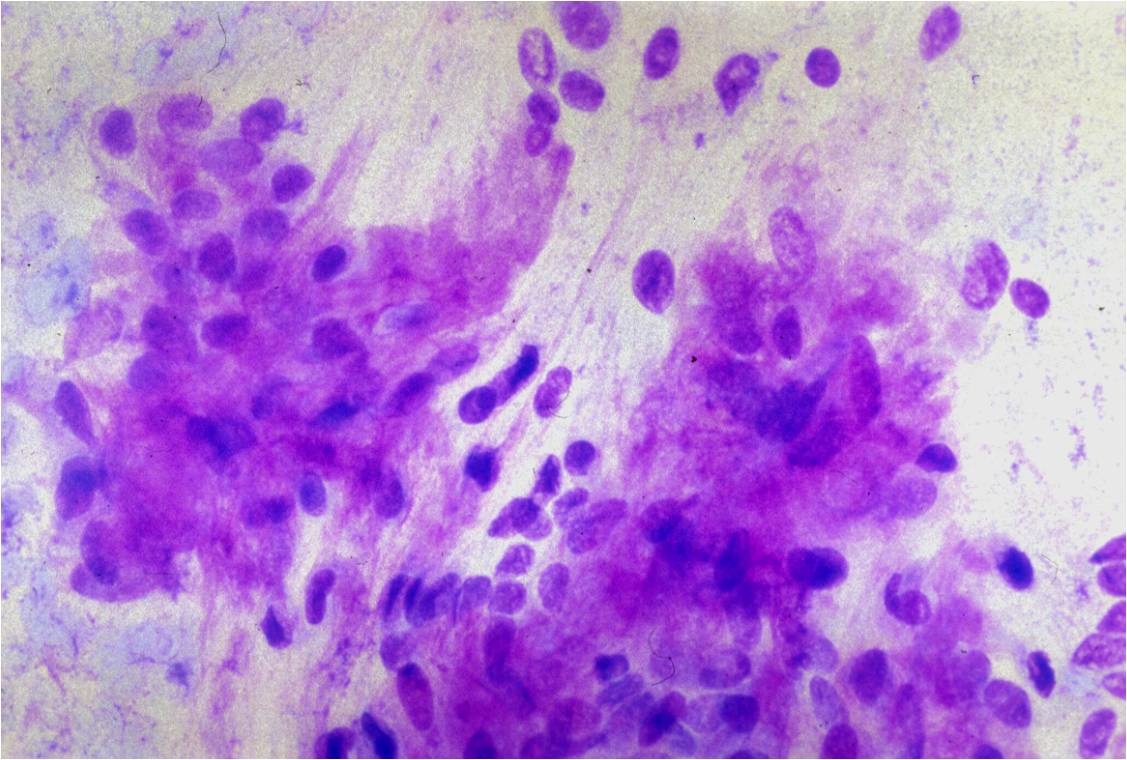 |
|
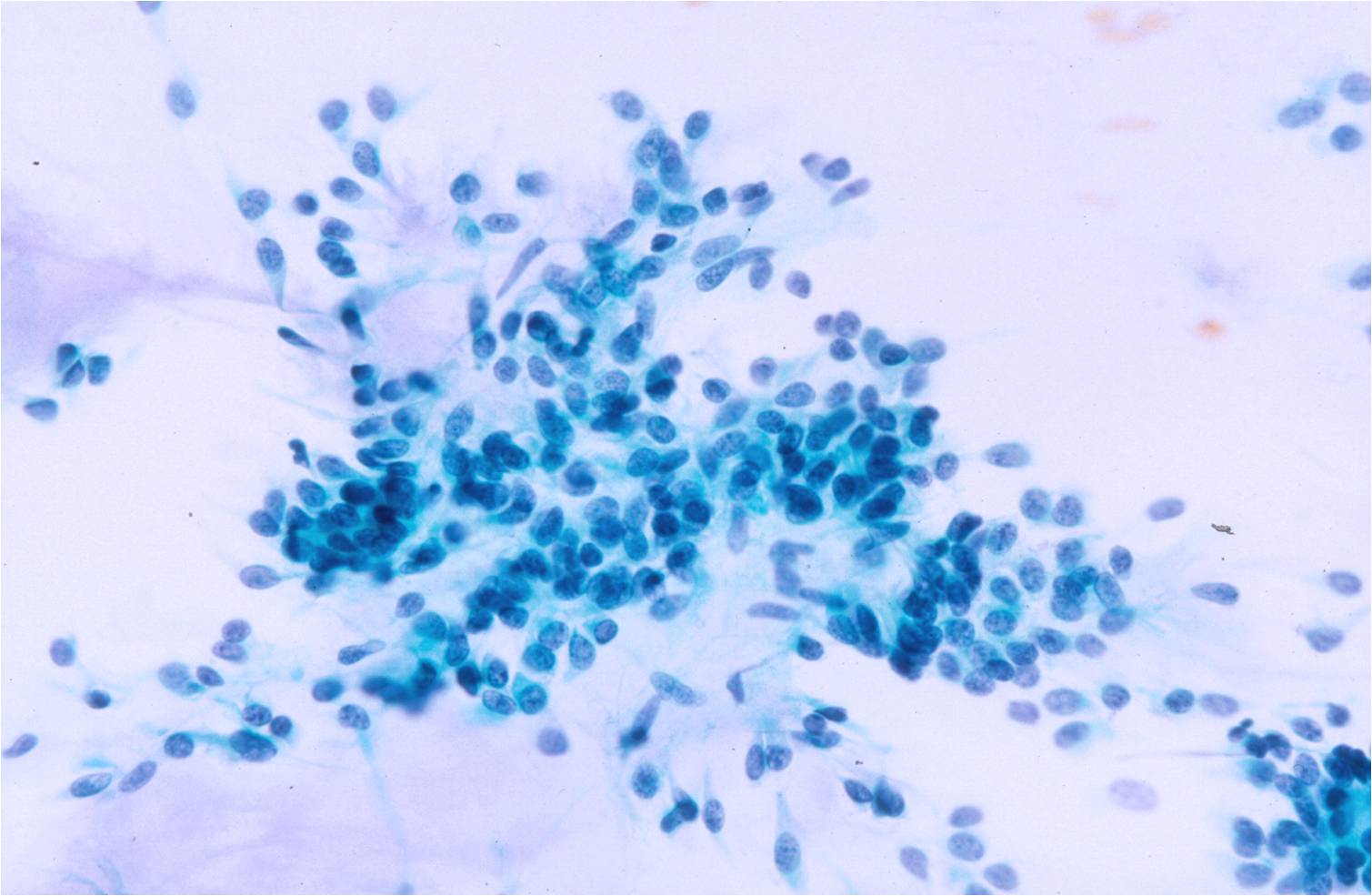 |
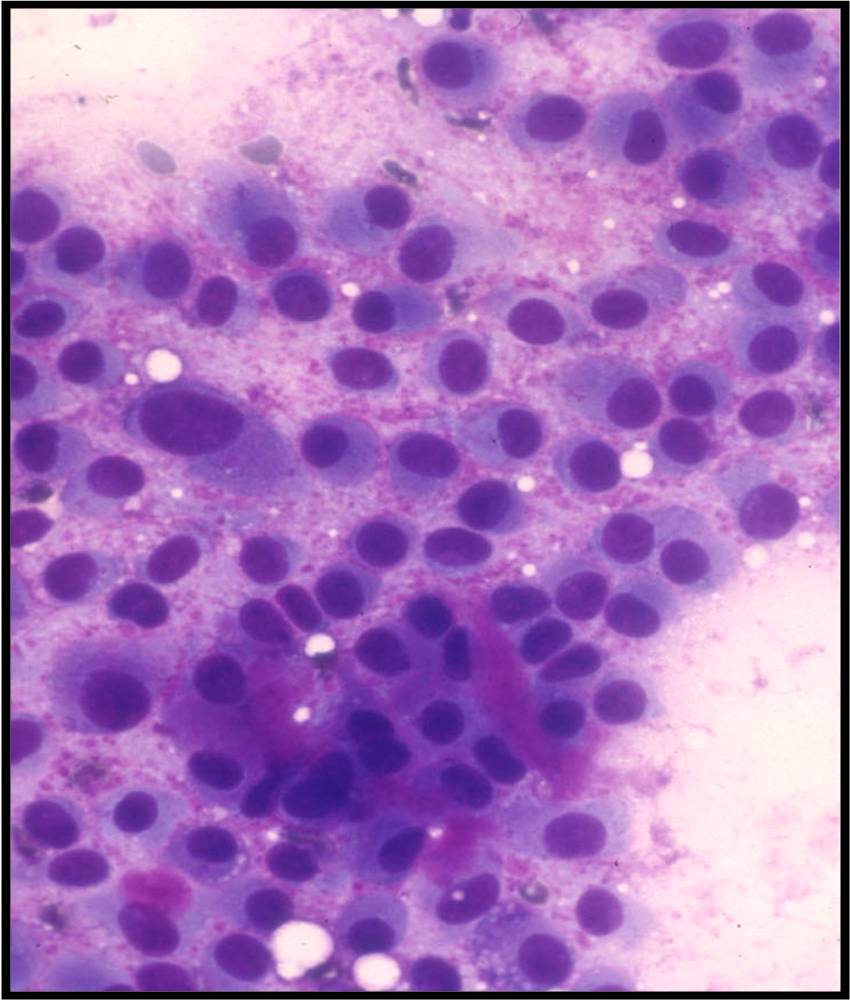 |
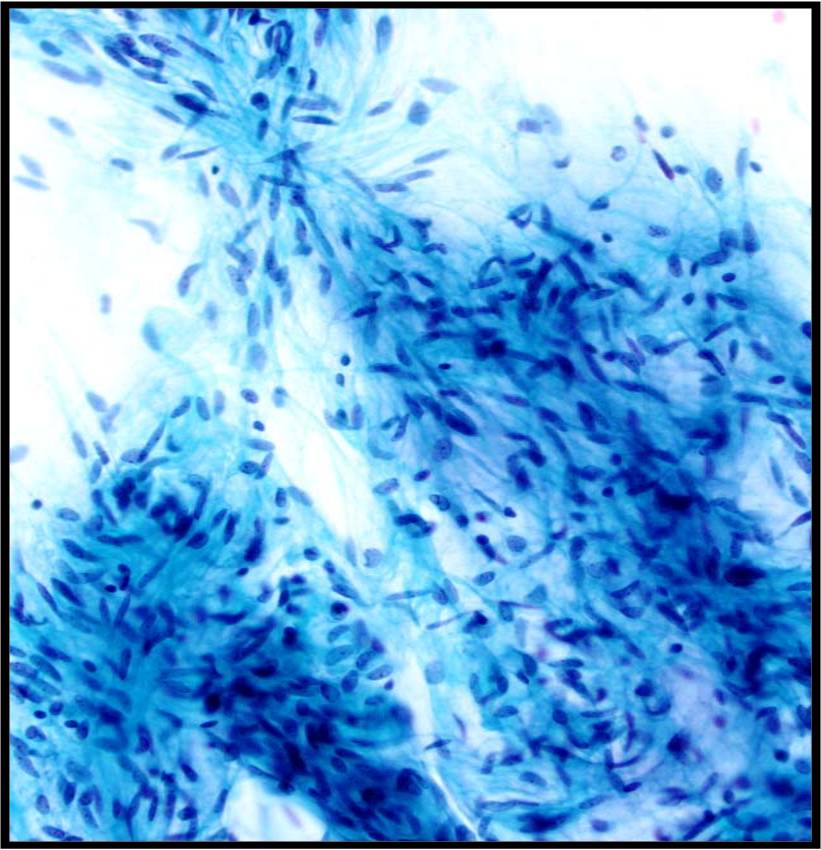 |
Warthin Tumor –SG9-12
- 5-10% of salivary gland tumors, most often in tail of parotid gland, sometimes bilateral
- Cystic dark fluid on aspiration
- History of slow growing mass
- Monolayered sheets of oncocytes with distinct cell borders and background lymphocytes and mast cells
- Lymphocytes my be in aggregates or spread in background of smears
- May see squamous metaplastic cells surrounding tumor
Adenoid Cystic Carcinoma – SG5-21
- 3-5% salivary gland tumors, common in minor and submandibular glands, malignant
- Increased cellularity!
- Crowded acellular 3D sheets of cribriform structures
- Finger or cup-shaped groups an important feature
- Small, round, uniform, bland basaloid cells with high N/C ratio, nuclear molding, scant cytoplasm and small distinct nucleoli surrounding mucoid balls, DQ stains red balls of mucopolysaccharide
- Necrosis present in 50% of cases
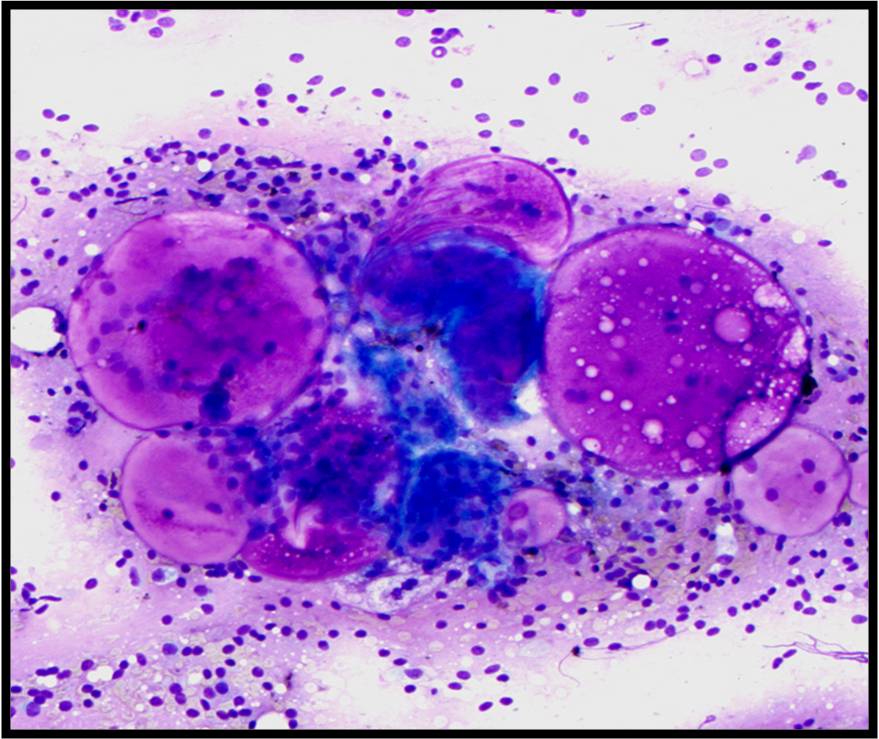 |
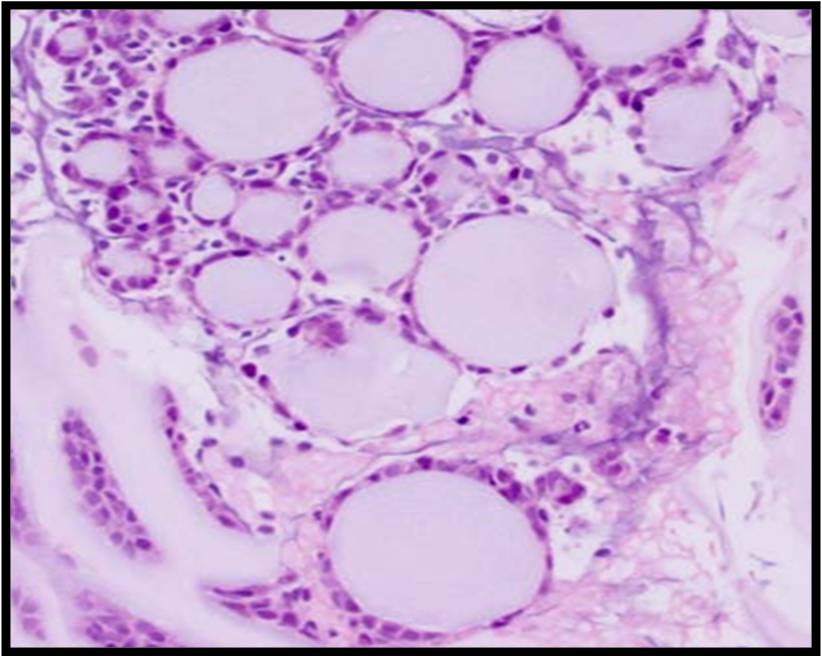 |
|
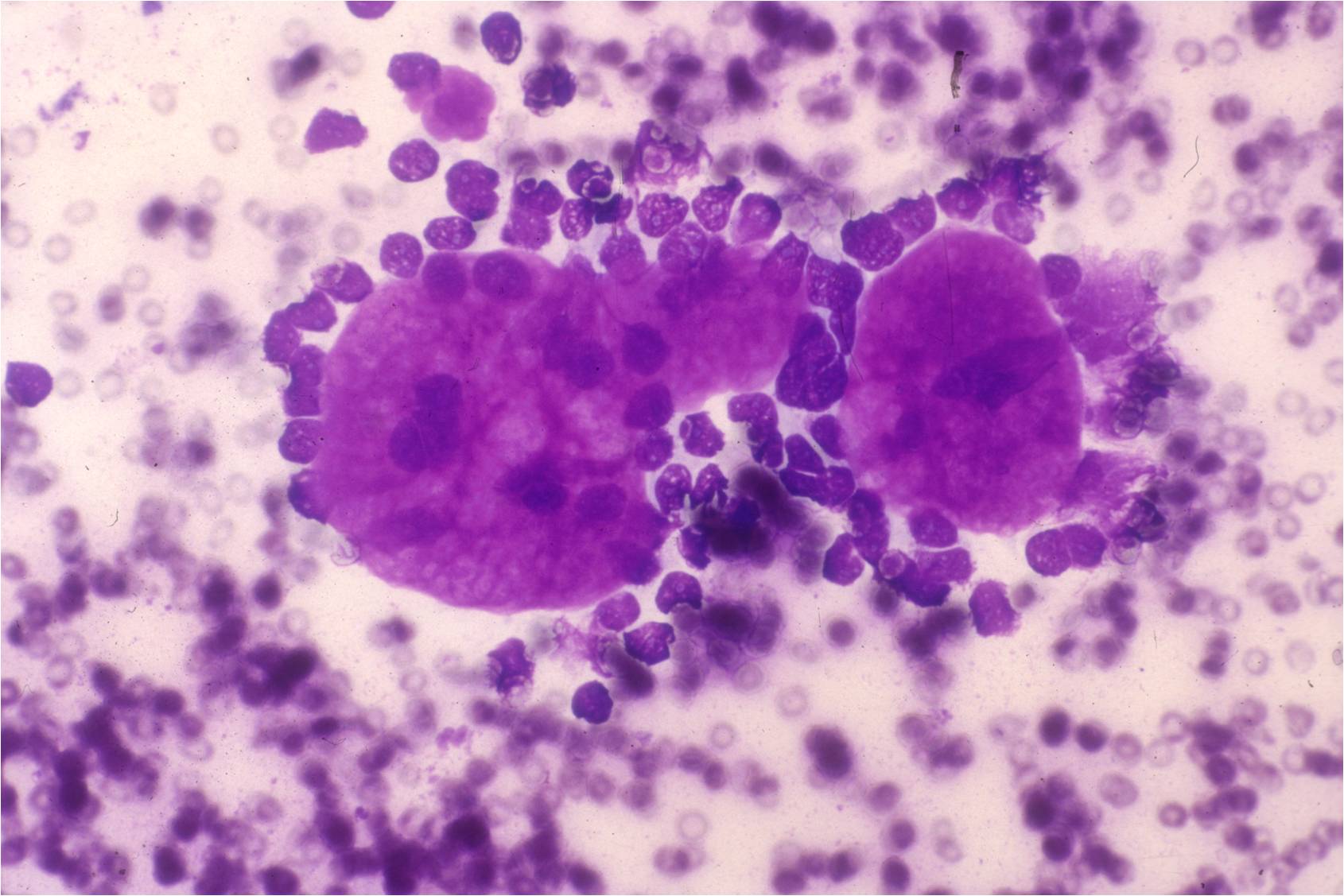 |
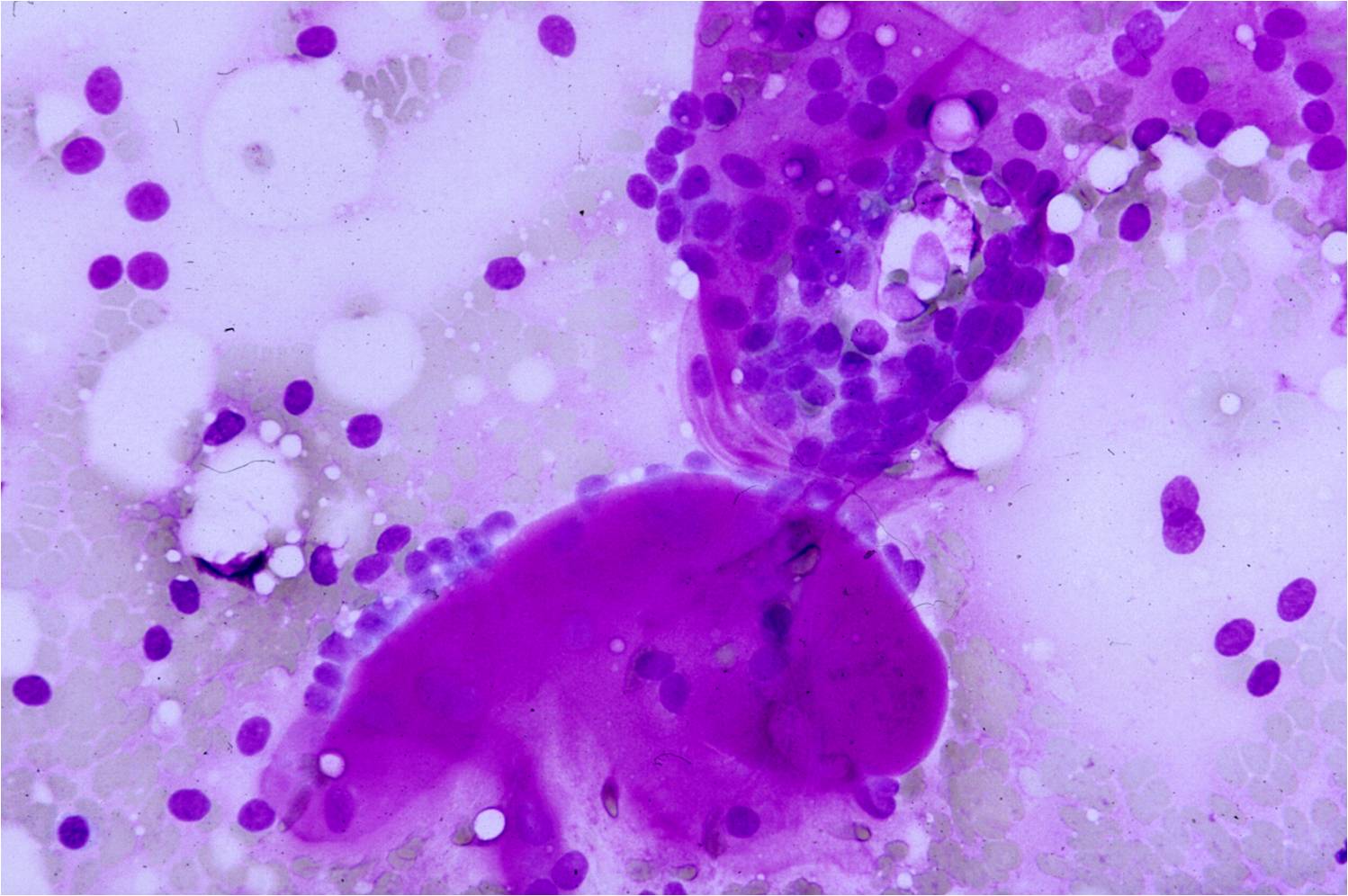 |
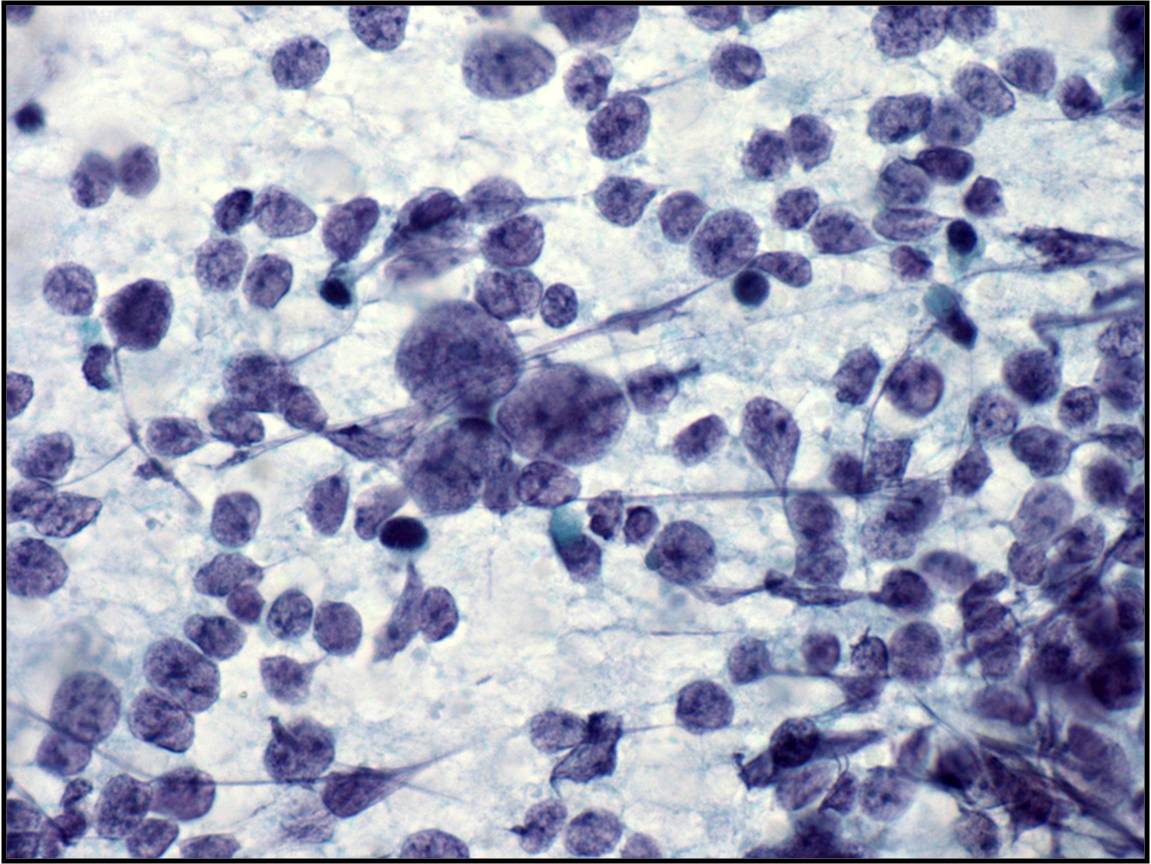 |
Head and Neck
Branchial Cleft Cyst (lymphoepithelial cyst) – SG-04
- Thick yellow fluid on aspiration
- Lateral cyst--occurs along anterior border of sternocleidomastoid muscle (ear to clavicle)
- Can occur in parotid in HIV patients
- Occasionally bilateral
- History of painless, firm mass with rapid growth; DDX: SCCA=may require a biopsy to confirm!
- Cysts lined by squamous cells, glandular cells or both cell types
- Epithelium surrounded by dense lymphocytic infiltrate
- Nucleated and anucleated squamous cells with keratinization shed into cystic space
- Acute and chronic inflammation
- Dirty granular background
Cytology service page
Warning: Display title "1-10 Liver Cytology: M Pitman MD, J Misdraji MD, Marilyn Nutter CT" overrides earlier display title "1-9 Salivary Gland and Head and Neck Cases: W.C. Faquin MD PhD, Lisa Ring CT".
The role of fine needle aspiration biopsy (FNAB) in the evaluation of focal liver lesions has evolved bringing with it new challenges.1,2 Advances in dynamic imaging modalities have obviated the need for tissue confirmation in clinically classic cases of hepatocellular carcinoma (HCC).3-12 Ultrasound (US) surveillance and serum alpha-fetoprotein (AFP) assessment of high-risk patients have resulted in the increasing detection of smaller and smaller nodules (≤ 2 cm size).13-16 Accurate cytohistologic characterization of small well-differentiated hepatocellular nodules on limited tissue samples is extremely challenging with therapeutic implications.
Recent years have seen much progress in genomic, microRNA profiling and proteomic studies for the molecular characterization of tumor and peritumoral tissues. They are emerging as promising tools not only in early tumor diagnosis but also for the prediction of HCC behavior in terms of prognosis and progression of disease, and in the development of personalized molecular targeted anticancer therapy and chemopreventive strategies.17-26 As FNAB is currently the most minimally invasive tool for tissue procurement, it is envisaged that FNAB will become a point of care in the management algorithm of HCC for diagnostic, therapeutic and prognostication purposes.27
Guidance Systems
Percutaneous (transabdominal) FNAB performed under ultrasound (US) or computed tomography (CT) guidance is a safe, efficacious and cost-effective outpatient procedure for diagnosis of focal liver lesions.28-30 Aspiration can also be performed at laparotomy or laparoscopy under palpation and/or direct vision. Endoscopic ultrasound-guided FNA (EUS-FNA) is the latest diagnostic and staging tool used primarily for left hepatic lobe lesions and hilar/perihilar masses with the needle traversing the gastrointestinal wall.31 Factors influencing the choice of guidance system include size and location of lesion, operator expertise and preference, and availability of imaging technologies.27-29
Ultrasound provides for rapid localization, flexible patient positioning, and variable imaging of the lesion without radiation. It is generally used for initial guidance, particularly with multiple lesions and/or large, relatively superficial lesions. Computed tomography allows for optimal resolution of small lesions or lesions not visible with US; accurate localization of the needle tip immediately prior to sampling; improved definition of tissue components and vascularity; and more precise demonstration of the anatomic relationships of a given lesion.
EUS-FNA is a safe, accurate and versatile technique but is highly operator-dependent.32-36 It allows for concurrent sampling of pancreas and liver lesions, confirming primary and metastatic malignancy in one single diagnostic encounter. The technique is useful for small and deep-seated left lobe lesions below CT/MRI resolution or not easily accessible by percutaneous FNAB. As such, it enhances staging of liver metastases; is good for early detection of multifocal HCC in cirrhosis; and for assessing the accurate number of lesions (intrahepatic staging of HCC) for transplantation eligibility.37,38
Percutaneous FNAB techniques include individual puncture, coaxial biopsy, and tandem needle biopsy technique.39 Multiple aspirations (up to four passes) can usually be performed with minimal morbidity. The needle size is between 20 to 22G. Aspiration needles with guillotine mechanism enable microbiopsy cores to be procured at the same sitting. Concomitant core needle (18G) biopsies may also be performed. Indications, Contraindications and Complications are outlined in Table 1.
Indications: FNAB is the diagnostic procedure of choice for focal liver lesions, especially to confirm a suspected malignancy. It is particularly advantageous for advanced malignancies and poor surgical candidates. Another indication is drainage of a cyst or abscess for culture and therapeutic ablation.40 An early affirmative diagnosis leads to cost savings in further investigational tests and hospitalization.
Contraindications: Contraindications for percutaneous FNAB include: (i) an uncorrectable bleeding diathesis; (ii) lack of a safe access route, i.e. biopsy through a vascular structure; and (iii) an uncooperative patient in which the need for awkward positioning or maintenance of strict breath control is necessary to assure proper needle placement.28 For EUS-FNA, gastrointestinal obstruction is an absolute contraindication due to risk of perforation.34
Complications: Complications of FNAB are uncommon.39 There may be bleeding - mostly associated with severe cirrhosis with coagulopathy; size of needle used particularly in vascular lesions; and large superficial tumors not covered by normal parenchyma.12 Needle tract seeding is extremely rare (incidence of 0.003 - 0.009% for malignancy in general; and 0.003 - 5% for HCC; if a small (22G), non-cutting needle is used the rate for HCC is around 0.11%).11,41-44 A higher incidence of HCC recurrence in the transplanted liver has been reported in cases with preoperative FNAB.45 Mortality, usually due to bleeding, is rare with a reported rate of 0.018%.42
Clinical Considerations
Much debate surrounds the preoperative FNAB diagnosis of HCC.27
Reasons cited for opposing FNAB include:46-48
• Advances in dynamic imaging modalities are sufficiently sensitive for establishing an HCC diagnosis 3,8,10,11,49
• Risk of needle tract seeding 11,41-44,50
• Risk of intraperitoneal bleeding 12
• Risk of intraprocedural hematogenous dissemination resulting in higher incidence of tumor recurrence and post-transplantation recurrence 41,45
• Adoption of the “wait and see” policy for hepatocellular nodules <1 cm 4,5
• Indeterminate cytohistologic reports rendered for well-differentiated hepatocellular nodules 51,52
Reasons cited for favoring FNAB include:53,54
• Serum alpha-fetoprotein has low sensitivity (<50%) 55,56
• Use of coaxial technique of biopsy may reduce risk of seeding 57
• To cut down on costs of long-term imaging surveillance
• To avoid a futile transplantation in false positive imaging cases 7
• To allay patient anxiety once a liver nodule has been detected on imaging
• Confirmation of HCC increases eligibility for liver transplantation
• For immediate institution of anti-HCC therapy when the lesion is still <2 cm
According to the European Association for the Study of Liver Diseases 2000 Conference (EASL) and American Association for the Study of Liver Disease (AASLD) guidelines, nodules >2 cm in cirrhotic livers are diagnosed as HCC if they show intense arterial profile with contrast washout in delayed venous phase on one dynamic imaging modality.4,5 Nodules between 1 and 2 cm in cirrhotic livers require concurrence of two coincidental imaging modalities; otherwise, biopsy is recommended.49 For nodules <1 cm, the EASL guidelines recommend the “wait and see” policy with 3-monthly US surveillance. It should be noted that about 68% of the nodules <1 cm in cirrhotic livers turn out to be HCCs.58 Ultrasound-guided FNAB of subcentimeter hepatic nodules in skilled hands with expert reader yield correct diagnoses in 90% of cases.41 The conundrum is to balance the risk of unnecessary surgery (2.5%) against the risk of seeding.9,11,53,54
Excerpted from Wee and Pitman. Liver Cytology. Surgical Pathology of the GI Tract, Liver, Biliart Tract and Pancreas. Odze and Goldblum, eds.
1. Wee A. Fine needle aspiration biopsy of hepatocellular carcinoma and hepatocellular nodular lesions: Role,
controversies and approach to diagnosis. Cytopathology 2011;22:287-305.
2. Fassina A. “The map is not the territory”: FNA the map and liver the territory. Cytopathology 2011;22:285-286.
3. Baron RL, Brancatelli G: Computed tomographic imaging of hepatocellular carcinoma. Gastroenterology 2004; 127:S133-143.
4. Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodes J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001; 35:421-430.
5. Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005;42:1208-1236.
6. Caturelli E, Ghittoni G, Roselli P, De Palo M, Anti M. Fine needle biopsy of focal liver lesions: the hepatologist’s point of view. Liver Transpl 2004;10 (2 Suppl 1):S26-29.
7. Durand F,Belghiti J,Paradis V.Liver transplantation for hepatocellular carcinoma: role of biopsy.Liver Transpl 2007;13 (11 Suppl 2):S17-23.
8. Saar B, Kellner-Weldon F. Radiological diagnosis of hepatocellular carcinoma. Liver Int 2008; 28:189-199.
9. Schőlmerich J, Schacherer D.Diagnostic biopsy for hepatocellular carcinoma in cirrhosis: useful, necessary, dangerous, or academic sport? Gut 2004;53:1224-1226.
10. Taouli B, Losada M, Holland A, Krinsky G. Magnetic resonance imaging of hepatocellular carcinoma. Gastroenterology 2004; 127:S144-152.
11. Torzilli G, Minagawa M, Takayama T et al. Accurate preoperative evaluation of liver mass lesions without fine-needle biopsy. Hepatology 1999;30:889–893.
12. Wang P, Meng ZQ, Chen Z et al. Diagnostic value and complications of fine needle aspiration for primary liver cancer and its influence on the treatment outcome. A study based on 3011 patients in China. Eur J Surg Oncol 2008;34:541-546.
13. Daniele B, Bencivenga A, Megna AS, Tinessa V. Alpha-fetoprotein and ultrasonography screening for hepatocellular carcinoma. Gastroenterology 2004; 127:S108-112.
14. D’Onofrio M, Faccioli N, Zamboni G, Malago R, Caffarri S, Fattovich G, Mucelli RP. Focal liver lesions in cirrhosis: value of contrast-enhanced ultrasonography compared with Doppler ultrasound and alpha-fetoprotein levels. Radiol Med 2008;113:978-991.
15. Llovet JM, Burroughs A, Bruix J, Hepatocellular carcinoma. Lancet 2003; 362:1907-1917.
16. Bolondi L, Screening for hepatocellular carcinoma in cirrhosis. J Hepatol 2003; 39:1076-1084.
17. Hoshida Yet al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med, 2008;359:1995-2004.
18. Ladeiro Y, Couchy G, Balabaud C, Bioulac-Sage P, Pelletier L, Rebouissou S, Zucman-Rossi J.MicroRNA profiling in hepatocellular tumors is associated with clinical features and oncogene/tumor suppressor gene mutations. Hepatology 2008;47:1955-1963
19. Lee JS, Chu IS, Heo J, Calvisi DF, Sun Z, Roskams T, Durnez A, Demetris AJ, Thorgeirsson SS. Classification and prediction of survival in hepatocellular carcinoma by gene expression profiling. Hepatology 2004;40:667-676.
20. Llovet JM et al. A molecular signature to discriminate dysplastic nodules from early hepatocellular carcinoma in HCV cirrhosis. Gastroenterology 2006;131: 1758-1767.
21. Malaguarnera G, Giordano M, Paladina I, Berretta M, Cappellani A, Malaguarnera M. Serum markers of hepatocellular carcinoma. Dig Dis Sci 2010; 55:2744-2755.
22. Roncalli M, Park YN, Di Tommaso L. Histopathological classification of hepatocellular carcinoma.Dig Liver Dis 2010;42 (Suppl 3):S228–234.
23. Roskams T, Kojiro M.Pathology of early hepatocellular carcinoma: conventional and molecular diagnosis.Semin Liver Dis 2010;30:17-25.
24. Schroder PC, Segura V, Riezu JI et al. A signature of six genes highlights defects on cell growth and specific metabolic pathways in murine and human hepatocellular carcinoma. Funct Integr Genomics 2011;11:419-429.
25. Wörns M, Galle P. Future perspectives in hepatocellular carcinoma. Dig Liver Dis2010;42 (Suppl 3):S302-309.
26. Yamashita T, Forgues M, Wang W, Kim JW, Ye Q, Jia H, Budhu A, Zanetti KA, Chen Y, Qin LX, Tang ZY, Wang XW. EpCAM and alpha-fetoprotein expression defines novel prognostic subtypes of hepatocellular carcinoma. Cancer Res 2008;68:1451-1461.
27. Wee A. Fine needle aspiration biopsy of hepatocellular carcinoma and related hepatocellular nodular lesions in cirrhosis: Controversies, challenges, and expectations. Patholog Res Int 2011;2001:587936. Epub 2011 Jun 30.
Guidance systems
28. Moulton JS, Leoni CJ, Quarfordt SD, Worth S. Percutaneous Image-Guided Biopsy. In: Baum S, Pentecost MJ, editors. Abrams' Angiography Interventional Radiology. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2006. p. 257-279.
29. Pitman MB, Szyfelbein WM. Fine Needle Aspiration Biopsy of the Liver. Boston: Butterworth-Heinemann; 1994.
30. Bakshi P, Srinivasan R, Rao KLet al. Fine needle aspiration biopsy in pediatric space-occupying lesions of liver: a retrospective study evaluating its role and diagnostic efficacy. J Pediatr Surg 2006;41:1903-1908.
31. Fritscher-Ravens A, Broering DC, Sriram PVet al. EUS-guided fine-needle aspiration cytodiagnosis of hilar cholangiocarcinoma: a case series. Gastrointest Endosc 2000;52:534-540.
32. Crowe DR, Eloubeidi MA, Chhieng DCet al.Fine-needle aspiration biopsy of hepatic lesions: computerized tomographic-guided versus endoscopic ultrasound guided FNA. Cancer 2006;108:180-185.
33. DeWitt J, LeBlanc J, McHenry Let al. Endoscopic ultrasound-guided fine needle aspiration cytology of solid liver lesions: a large single-center experience. Am J Gastroenterol 2003;98:1976-1981.
34. Quirk DM, Brugge WR. Endoscopic Ultrasonography-Directed Fine Needle Aspiration. In: Centeno BA, Pitman MB, editors. Fine Needle Aspiration Biopsy of the Pancreas. Boston: Butterworth-Heinemann; 1999. p. 13-16.
35. Singh P, Erickson RA, Mukhopadhyay Pet al. EUS for detection of the hepatocellular carcinoma: results of a prospective study. Gastrointest Endosc 2007;66:265-273.
36. Singh P, Mukhopadhyay P, Bhatt Bet al.Endoscopic ultrasound versus CT scan for detection of the metastases to the liver: results of a prospective comparative study. J Clin Gastroenterol 2009;43:367-373.
37. Awad SS, Fagan S, Abudayyeh Set al. Preoperative evaluation of hepatic lesions for the staging of hepatocellular and metastatic liver carcinoma using endoscopic ultrasonography. Am J Surg 2002;184:601-604.
38. Maheshwari A, Kantsevoy S, Jagannath S, Thuluvath PJ. Endoscopic ultrasound and fine-needle aspiration for the diagnosis of hepatocellular carcinoma. Clin Liver Dis 2010;14:325-332.
39. Strassburg CP, Manns MP. Approaches to liver biopsy techniques--revisited. Semin Liver Dis 2006;26:318-327.
Indications, contraindications, complications
40. Blonski WC, Campbell MS, Faust T, Metz DC. Successful aspiration and ethanol sclerosis of a large, symptomatic, simple liver cyst: case presentation and review of the literature. World J Gastroenterol 2006;12:2949-2954.
41. Durand F, Regimbeau JM, Belghiti J et al. Assessment of the benefits and risks of percutaneous biopsy before surgical resection of hepatocellular carcinoma. J Hepatol 2001;35:254-258.
42. Fornari F, Civardi G, Cavanna L et al. Complications of ultrasonically guided fine-needle abdominal biopsy. Results of a multicenter Italian study and review of the literature. The Cooperative Italian Study Group. Scand J Gastroenterol 1989;24:949-955.
43. Stigliano R, Marelli L, Yu D et al. Seeding following percutaneous diagnostic and therapeutic approaches for hepatocellular carcinoma. What is the risk and the outcome? Seeding risk for percutaneous approach of HCC. Cancer Treat Rev 2007;33:437-447.
44. Tung WC, Huang YJ, Leung SW et al. Incidence of needle tract seeding and responses of soft tissue metastasis by hepatocellular carcinoma postradiotherapy. Liver Int 2007;27:192-200.
45. Saborido BP, Diaz JC, de Los Galanes SJ et al. Does preoperative fine needle aspiration-biopsy produce tumor recurrence in patients following liver transplantation for hepatocellular carcinoma? Transplant Proc 2005;37:3874-3877.
Controversies
46. Cresswell AB, Welsh FKS, Ree M. A diagnostic paradigm for resectable liver lesions: to biopsy or not to biopsy? HPB (Oxford). 2009;11: 533-540.
47. Hemming AW, Cattral MS, Reed AI et al. Liver transplantation for hepatocellular carcinoma. Ann Surg 2001;233:652-659.
48. Stigliano R, Burroughs AK. Should we biopsy each liver mass suspicious for HCC before liver transplantation? – no, please don’t. J Hepatol 2005;43:563-568.
49. Forner A, Vilan R, Ayuso C, Bianchi L, Sole M, Ayuso JR, Boix L, Sala M, Varela M, Llovet JM, Bru C, Bruix J. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 2008;47:97-104.
50. Aldahham A, Boodai S, Alfuderi A, Almosawi A, Asfer S. Abdominal wall implantation of hepatocellular carcinoma. World J Surg Oncol 2006;4:72.
51. de Boer WB, Segal A, Frost FA, Sterrett GF. Cytodiagnosis of well differentiated hepatocellular carcinoma: can indeterminate diagnoses be reduced? Cancer (Cancer Cytopathol) 1999;87:270-277.
52. Wee A, Nilsson B. Highly well differentiated hepatocellular carcinoma and benign hepatocellular lesions. Can they be distinguished on fine needle aspiration biopsy? Acta Cytol 2003;47:16-26.
53. Chapoutot C, Perney P, Fabre D et al. Needle-tract seeding after ultrasound-guided puncture of hepatocellular carcinoma. A study of 150 patients. Gastroenterol Clin Biol 1999;23:552–556.
54. Ng KK, Poon RT, Lo CM et al. Impact of preoperative fine-needle aspiration cytologic examination on clinical outcome in patients with hepatocellular carcinoma in a tertiary referral center. Arch Surg 2004;139:193–200.
55. Forner A, Reig M, Bruix J. Alpha-fetoprotein for hepatocellular carcinoma diagnosis: The demise of a brilliant star. Gastroenterology 2009;137:26-29.
56. Sherman M: Alphafetoprotein: an obituary. J Hepatol 2001; 34:603-605.
57. Maturen KE, Nghiem HV, Marrero JA et al. Lack of tumor seeding of hepatocellular carcinoma after percutaneous needle biopsy using coaxial cutting needle technique. AJR Am J Roentgenol 2006;187:1184-1187.
58. Caturelli E, Solmi L, Anti M et al. Ultrasoundguided fine needle biopsy of early hepatocellular carcinoma complicatingliver cirrhosis: a multicentre study. Gut 2004;53:1356-1362.
Procurement Methods for Liver FNAB
Guidance Systems
Percutaneous (transabdominal) FNAB performed under ultrasound (US) or computed tomography (CT) guidance is a safe, efficacious and cost-effective outpatient procedure for diagnosis of focal liver lesions.28-30 Aspiration can also be performed at laparotomy or laparoscopy under palpation and/or direct vision. Endoscopic ultrasound-guided FNA (EUS-FNA) is the latest diagnostic and staging tool used primarily for left hepatic lobe lesions and hilar/perihilar masses with the needle traversing the gastrointestinal wall.31 Factors influencing the choice of guidance system include size and location of lesion, operator expertise and preference, and availability of imaging technologies.27-29
Ultrasound provides for rapid localization, flexible patient positioning, and variable imaging of the lesion without radiation. It is generally used for initial guidance, particularly with multiple lesions and/or large, relatively superficial lesions. Computed tomography allows for optimal resolution of small lesions or lesions not visible with US; accurate localization of the needle tip immediately prior to sampling; improved definition of tissue components and vascularity; and more precise demonstration of the anatomic relationships of a given lesion.
EUS-FNA is a safe, accurate and versatile technique but is highly operator-dependent.32-36 It allows for concurrent sampling of pancreas and liver lesions, confirming primary and metastatic malignancy in one single diagnostic encounter. The technique is useful for small and deep-seated left lobe lesions below CT/MRI resolution or not easily accessible by percutaneous FNAB. As such, it enhances staging of liver metastases; is good for early detection of multifocal HCC in cirrhosis; and for assessing the accurate number of lesions (intrahepatic staging of HCC) for transplantation eligibility.37,38
Percutaneous FNAB techniques include individual puncture, coaxial biopsy, and tandem needle biopsy technique.39 Multiple aspirations (up to four passes) can usually be performed with minimal morbidity. The needle size is between 20 to 22G. Aspiration needles with guillotine mechanism enable microbiopsy cores to be procured at the same sitting. Concomitant core needle (18G) biopsies may also be performed. Indications, Contraindications and Complications are outlined in Table 1.
Indications: FNAB is the diagnostic procedure of choice for focal liver lesions, especially to confirm a suspected malignancy. It is particularly advantageous for advanced malignancies and poor surgical candidates. Another indication is drainage of a cyst or abscess for culture and therapeutic ablation.40 An early affirmative diagnosis leads to cost savings in further investigational tests and hospitalization.
Contraindications: Contraindications for percutaneous FNAB include: (i) an uncorrectable bleeding diathesis; (ii) lack of a safe access route, i.e. biopsy through a vascular structure; and (iii) an uncooperative patient in which the need for awkward positioning or maintenance of strict breath control is necessary to assure proper needle placement.28 For EUS-FNA, gastrointestinal obstruction is an absolute contraindication due to risk of perforation.34
Complications: Complications of FNAB are uncommon.39 There may be bleeding - mostly associated with severe cirrhosis with coagulopathy; size of needle used particularly in vascular lesions; and large superficial tumors not covered by normal parenchyma.12 Needle tract seeding is extremely rare (incidence of 0.003 - 0.009% for malignancy in general; and 0.003 - 5% for HCC; if a small (22G), non-cutting needle is used the rate for HCC is around 0.11%).11,41-44 A higher incidence of HCC recurrence in the transplanted liver has been reported in cases with preoperative FNAB.45 Mortality, usually due to bleeding, is rare with a reported rate of 0.018%.42
Excerpted from Wee and Pitman. Liver Cytology. Surgical Pathology of the GI Tract, Liver, Biliart Tract and Pancreas. Odze and Goldblum, eds.
Guidance systems
1. Moulton JS, Leoni CJ, Quarfordt SD, Worth S. Percutaneous Image-Guided Biopsy. In: Baum S, Pentecost MJ, editors. Abrams' Angiography Interventional Radiology. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2006. p. 257-279.
2. Pitman MB, Szyfelbein WM. Fine Needle Aspiration Biopsy of the Liver. Boston: Butterworth-Heinemann; 1994.
3. Bakshi P, Srinivasan R, Rao KLet al. Fine needle aspiration biopsy in pediatric space-occupying lesions of liver: a retrospective study evaluating its role and diagnostic efficacy. J Pediatr Surg 2006;41:1903-1908.
4. Fritscher-Ravens A, Broering DC, Sriram PVet al. EUS-guided fine-needle aspiration cytodiagnosis of hilar cholangiocarcinoma: a case series. Gastrointest Endosc 2000;52:534-540.
5. Crowe DR, Eloubeidi MA, Chhieng DCet al.Fine-needle aspiration biopsy of hepatic lesions: computerized tomographic-guided versus endoscopic ultrasound guided FNA. Cancer 2006;108:180-185.
6. DeWitt J, LeBlanc J, McHenry Let al. Endoscopic ultrasound-guided fine needle aspiration cytology of solid liver lesions: a large single-center experience. Am J Gastroenterol 2003;98:1976-1981.
7. Quirk DM, Brugge WR. Endoscopic Ultrasonography-Directed Fine Needle Aspiration. In: Centeno BA, Pitman MB, editors. Fine Needle Aspiration Biopsy of the Pancreas. Boston: Butterworth-Heinemann; 1999. p. 13-16.
8. Singh P, Erickson RA, Mukhopadhyay Pet al. EUS for detection of the hepatocellular carcinoma: results of a prospective study. Gastrointest Endosc 2007;66:265-273.
9. Singh P, Mukhopadhyay P, Bhatt Bet al.Endoscopic ultrasound versus CT scan for detection of the metastases to the liver: results of a prospective comparative study. J Clin Gastroenterol 2009;43:367-373.
10. Awad SS, Fagan S, Abudayyeh Set al. Preoperative evaluation of hepatic lesions for the staging of hepatocellular and metastatic liver carcinoma using endoscopic ultrasonography. Am J Surg 2002;184:601-604.
11. Maheshwari A, Kantsevoy S, Jagannath S, Thuluvath PJ. Endoscopic ultrasound and fine-needle aspiration for the diagnosis of hepatocellular carcinoma. Clin Liver Dis 2010;14:325-332.
12. Strassburg CP, Manns MP. Approaches to liver biopsy techniques--revisited. Semin Liver Dis 2006;26:318-327.
Indications, contraindications, complications
13. Blonski WC, Campbell MS, Faust T, Metz DC. Successful aspiration and ethanol sclerosis of a large, symptomatic, simple liver cyst: case presentation and review of the literature. World J Gastroenterol 2006;12:2949-2954.
14. Durand F, Regimbeau JM, Belghiti J et al. Assessment of the benefits and risks of percutaneous biopsy before surgical resection of hepatocellular carcinoma. J Hepatol 2001;35:254-258.
15. Fornari F, Civardi G, Cavanna L et al. Complications of ultrasonically guided fine-needle abdominal biopsy. Results of a multicenter Italian study and review of the literature. The Cooperative Italian Study Group. Scand J Gastroenterol 1989;24:949-955.
16. Stigliano R, Marelli L, Yu D et al. Seeding following percutaneous diagnostic and therapeutic approaches for hepatocellular carcinoma. What is the risk and the outcome? Seeding risk for percutaneous approach of HCC. Cancer Treat Rev 2007;33:437-447.
17. Tung WC, Huang YJ, Leung SW et al. Incidence of needle tract seeding and responses of soft tissue metastasis by hepatocellular carcinoma postradiotherapy. Liver Int 2007;27:192-200.
18. Saborido BP, Diaz JC, de Los Galanes SJ et al. Does preoperative fine needle aspiration-biopsy produce tumor recurrence in patients following liver transplantation for hepatocellular carcinoma? Transplant Proc 2005;37:3874-3877.
Test Platform and Processing
The types of small tissue samples from liver FNAB include direct smears, needle rinses, core imprints, cellblocks, microbiopsies and core needle biopsies. Smears may be air-dried and stained with Diff-Quik® and/or May-Grϋnwald-Giemsa (MGG), and/or fixed in 95% alcohol and stained by the Papanicolaou method. Rapid touch prep core imprints can be performed to assess adequacy of tissue cores.1 If well-fixed, adequately smeared slides are difficult to obtain, the aspirate can be expressed into a preservative and submitted as a liquid-based specimen for processing by either the ThinPrep® or Sure Path™ methods.
Cellblocks are made from needle rinses and tissue fragments.2,3 Particulate material should be quickly retrieved from the glass slides prior to staining for formalin-fixed paraffin-embedded cellblock preparation. Histologic sections allow for architectural appraisal, special stains and immunohistochemistry. Immunocytochemistry may be necessary if only smears are available.
Excerpted from Wee and Pitman. Liver Cytology. Surgical Pathology of the GI Tract, Liver, Biliart Tract and Pancreas. Odze and Goldblum, eds.
1. Hahn PF, Eisenberg PJ, Pitman MB, Gazelle GS, Mueller PR. Cytopathologic touch preparations (imprints) from core needle biopsies: accuracy compared with that of fine-needle aspirates. AJR 1995;165:1277-1279.
2. Ceyhan K, Kupana SA, Bektas Met al. The diagnostic value of on-site cytopathological evaluation and cell block preparation in fine-needle aspiration cytology of liver masses. Cytopathology 2006;17:267-274.
3. Wee A. Fine needle aspiration biopsy of the liver: Algorithmic approach and current issues in the diagnosis of hepatocellular carcinoma. Cytojournal 2005;2:7.
Reporting and Terminology
Focal liver lesions range from cystic and inflammatory/infectious entities to benign/malignant, primary/metastatic neoplasms. HCC is well-known for its heterogeneity. Intrahepatic cholangiocarcinoma (CC) frequently has nondescript adenocarcinoma features. The liver is a common depository for metastases. Given these permutations, one has to recognize that primary liver carcinomas can mimic many tumors, and vice versa. The diagnostic issues are listed below:
Diagnostic Issues in Fine Needle Aspiration Biopsy Evaluation of Focal Liver Lesions
• To distinguish well-differentiated hepatocellular nodules from reactive hepatocytes
• To distinguish between the various types of benign well-differentiated hepatocellular nodules
• To distinguish early well-differentiated hepatocellular carcinoma from benign hepatocellular nodules
• To distinguish poorly differentiated hepatocellular carcinoma from intrahepatic cholangiocarcinoma
• To distinguish intrahepatic cholangiocarcinoma from metastatic adenocarcinomas
• To distinguish poorly differentiated primary liver carcinomas from metastases
• To ascertain the histogenesis of a non-hepatocellular tumors
• To ascertain the primary site of origin of a malignant non-hepatocellular tumors
• To differentiate between benign and malignant cystic lesions
• To recognize inflammatory/infectious lesions that may mimic tumors
Integrative Approach to the Evaluation of Liver Aspirates
A stepwise algorithmic approach incorporating full clinicopathologic correlation is of utmost importance.1
Step 1: Evaluate clinical information
A patient may present with one or more focal liver lesions under the following scenarios: (i) Routine medical checkup, (ii) Screening or surveillance for chronic liver disease, (iii) Follow-up of known cancer case, (iv) Investigation of symptomatic patient, or (v) Investigation of pediatric lesion. Relevant clinical findings, liver function profile, serology and tumor markers should be sought.2-4
Step 2: Evaluate radiologic information
The focal liver lesion may be cystic or solid, single or multiple. Evidence of pre-existing or chronic hepatobiliary disease and other relevant associated radiologic findings should be sought. Advances in dynamic contrast-enhanced imaging modalities have increased the sensitivity and specificity for the detection of classic HCC. Siderotic nodules are readily detected on T2 -orT2* weighted MR imaging.3
Step 3: Evaluate cytohistologc findings
Scan the smears at low power to establish the pattern and architecture before closer scrutiny for cellular details as listed in Table 5. One should ascertain whether the lesion is: (i) cystic – true cyst or pseudocyst, benign or malignant; or (ii) solid – hepatocellular or non-hepatocellular, benign or malignant. The diagnostic algorithms for the evaluation of cystic and solid lesions of the liver are below:
Diagnostic Algorithm of Cystic Lesions of the Liver (modified from WHO Classification of Tumors of the Digestive System, 4th edition, 2010)4
I. Cystic lesion with epithelial lining
a. Cuboidal / low columnar epithelium: Solitary bile duct cyst, fibropolycystic disease and obstructive dilatation of bile duct
b. Ciliated epithelium: Ciliated foregut cyst
c. Biliary / mucinous / oncocytic (+/- papillae):
(i) With ovarian-like stroma
- Mucinous cystic neoplasm with low-, intermediate- or high-grade intraepithelial neoplasia
- Mucinous cystic neoplasm with an associated invasive carcinoma
(ii) Without ovarian-like stroma
- Biliary intraductal papillary neoplasm with low-, intermediate- or high-grade intraepithelial neoplasia
- Biliary intraductal papillary neoplasm with an associated invasive carcinoma
d. Intrahepatic cholangiocarcinoma associated with cystic change; arising from malignant transformation in pre-existing cystic disease; or associated with cystically dilated bile ducts
e. Metastases
II. Cystic lesion without epithelial lining
a. Neoplastic (including tumor-like lesions):
(i) Cavernous hemangioma
(ii) Mesenchymal hamartoma
(iii) Cystic degeneration in any benign or malignant tumor
(iv) Undifferentiated embryonal sarcoma
b. Nonneoplastic
(i) Laminated wall: Hydatid cyst
(ii) Inflammation and necrosis: Pyogenic / fungal / amebic abscess, granulomas, necrotizing eosinophilic granuloma, hydatid cyst
(iii) Hemorrhagic cyst: Cystic hematoma
Diagnostic Algorithm of Solid Lesions of the Liver
I. Hepatocellular appearance
a. Focal fatty change
b. Large regenerative nodule
c. Dysplastic nodule, low- or high-grade
d. Siderotic nodule (regenerative, dysplastic)
e. Focal nodular hyperplasia
f. Hepatocellular adenoma
g. Hepatocellular carcinoma, variants and special types
h. Hepatoblastoma
II. Hepatocellular and glandular appearance
a. Combined hepatocellular-cholangiocarcinoma
III. Non-hepatocellular appearance
a. Glandular pattern: Bile duct adenoma (peribiliary gland hamartoma), intrahepatic cholangiocarcinoma, combined hepatocellular-cholangiocarcinoma, metastases
b. Squamous, including adenosquamous, pattern: Intrahepatic cholangiocarcinoma, metastases
c. Mucinous pattern: Mucinous cystic neoplasm, intrahepatic cholangiocarcinoma, metastases
d. Papillary pattern: Intraductal papillary neoplasm, intrahepatic cholangiocarcinoma, metastases
e. Clear cell pattern: Angiomyolipoma, metastases (renal cell carcinoma, adrenocortical carcinoma)
f. Oncocytic cell pattern: Angiomyolipoma, metastases (renal cell carcinoma, adrenocortical carcinoma), melanoma
g. Small cell pattern: Neuroendocrine tumor, lymphoma, metastases (small cell carcinoma, lobular carcinoma of breast, melanoma), inflammatory pseudotumor
h. Large cell pattern: Neuroendocrine carcinoma, metastases (undifferentiated carcinoma)
i. Spindle cell pattern: Cavernous hemangioma, infantile hemangioma, solitary fibrous tumor, inflammatory pseudotumor, angiomyolipoma, gastrointestinal stromal tumor, sarcomatoid carcinoma (including primary liver carcinomas and metastatic sarcomatoid renal cell carcinoma), sarcoma (primary and metastatic, including angiosarcoma, Kaposi sarcoma, carcinosarcoma, hepatobiliary rhabdomyosarcoma, synovial sarcoma and leiomyosarcoma)
j. Giant cell pattern: Sarcomatoid/giant cell carcinoma (primary liver carcinomas and metastases), sarcoma (primary and metastases)
k. Others: Nodular hematopoiesis, epithelioid hemangioendothelioma, germ cell tumors (teratoma and yolk sac tumor)
Step 4: Evaluate ancillary studies
The initial cytomorphologic impression is crucial as the material available usually limits the choice of immunohistochemical and other ancillary tests.
Step 5: Correlate all of above
Clinicopathologic correlation is mandatory for the final definitive diagnosis. An indeterminate diagnosis may be rendered for highly well-differentiated hepatocellular nodules. Information gleaned from all the above, including a complete immunohistochemical workup, may only help establish a generic morphologic diagnosis for non-hepatocellular lesions but not the organ of origin.
Excerpted from Wee and Pitman. Liver Cytology. Surgical Pathology of the GI Tract, Liver, Biliart Tract and Pancreas. Odze and Goldblum, eds.
1. Wee A and P Sampatanukul. Fine needle aspiration cytology of the liver. Diagnostic Algorithms. A Southeast Asian perspective. Bangkok: Year Book Publisher Co., Ltd, 2004.
2. Zhang J, Krinsky GA. Iron-containing nodules of cirrhosis. NMR Biomed 2004;17:459-464.
3. Krinsky GA, Lee VS, Nguyen MT et al. Siderotic nodules at MR imaging: regenerative or dysplastic? J Comput Assist Tomogr 2000;24:773-776.
4. Bosman FT, Carneiro F, Hruban RH, Theise ND (Eds.). WHO Classification of Tumours of the Digestive System. IARC: Lyon 2010
Basic cytomorphology
Regenerative, Dysplastic and Benign Neoplastic Hepatocellular Nodules – N12-12133 reactive, N12-4918 and N12-6339
- Hepatocytes arranged in jagged irregular clusters, small clusters, short rows and singly (depending on regenerative, dysplastic or neoplastic nature)
- No peripheral endothelium
- Clusters rarely have transgressing endothelium (except for neoplastic aspirates)
- Reactive hepatocytes show sibling polymorphism with normal nuclear-to-cytoplasmic ratio (1/3) and frequent binucleation
- Sporadically placed large, atypical cells with mild pleomorphism of nuclear size but normal nuclear-to-cytoplasmic ratio (in dysplastic hepatocytes with large cell change)
- Small uniformly monotonous hepatocytes with increased nuclear-to-cytoplasmic ratio and nuclear crowding (in dysplastic hepatocytes with small cell change)
- Variably prominent nucleoli but no macroeosinophilic nucleoli
- Cytoplasm is generally abundant (except in small cell change) and granular but may show fatty change, lipofuscin pigment, or iron deposition
- Bile duct epithelium present (except in LCA)
- Core biopsies provide specific diagnosis: Reticulin stain shows retention of 1 to 2 cells thick hepatic plate framework on cellblock; unpaired arterioles in parenchyma for LCA; bile duct proliferation and scar for FNH
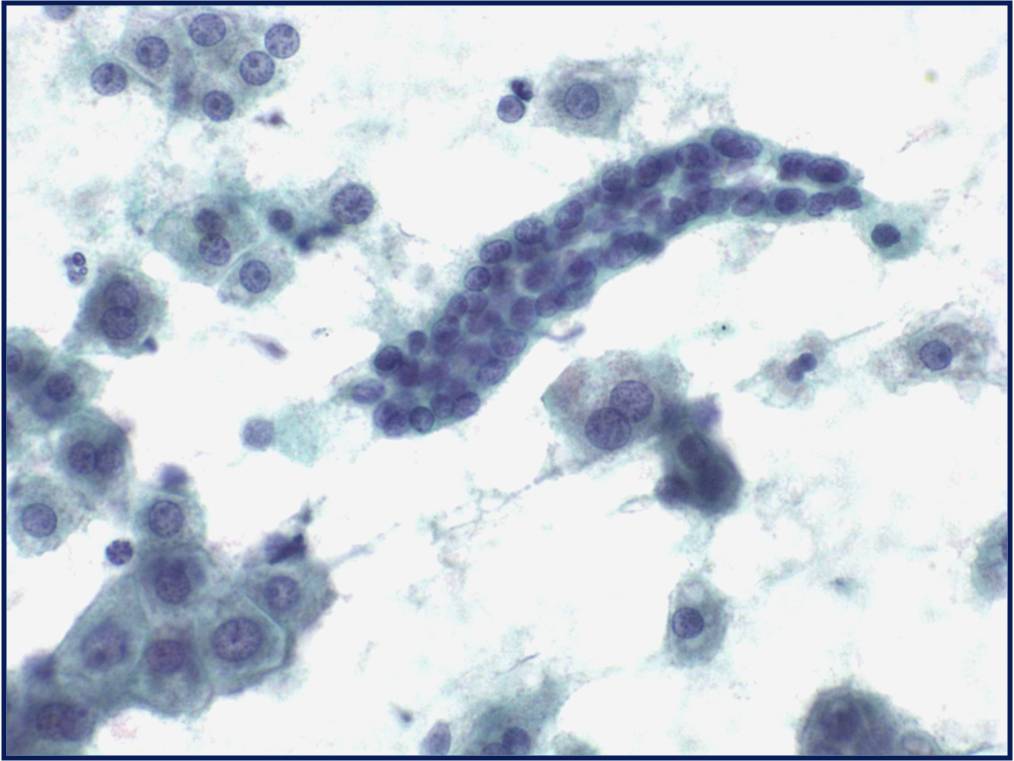 |
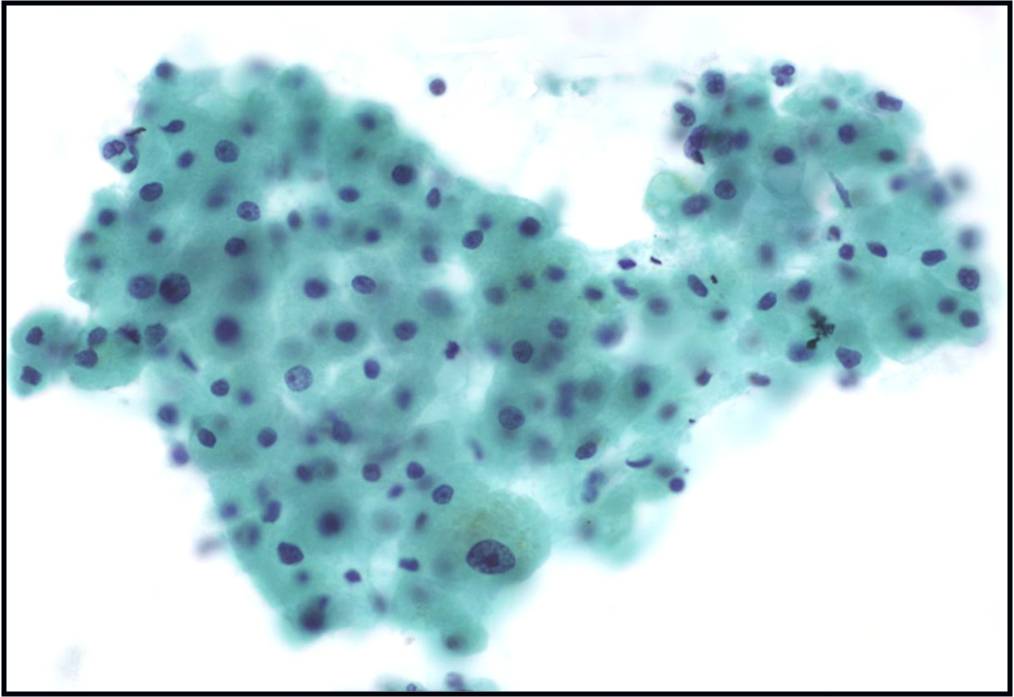 |
|
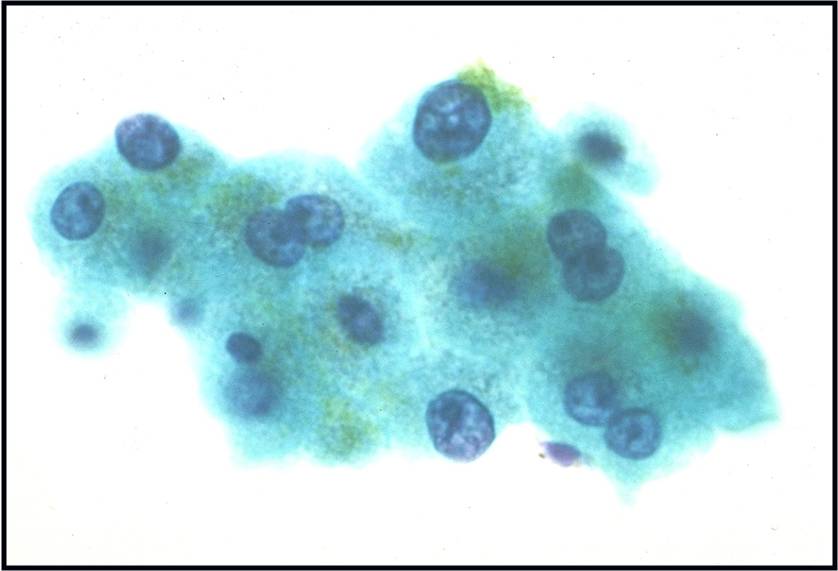 |
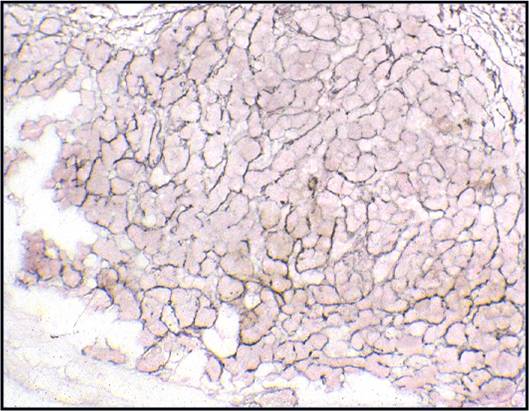 |
|
Well-differentiated Hepatocellular Carcinoma – N13-6335 and N12-5663
- Under low power microscopy, smear pattern shows trails of smooth-edged, arborizing clusters of thickened trabeculae with peripheral endothelium (pathognomonic)
- Under low power, smear pattern shows many loosely cohesive sheets of hepatocytes with transgressing vessels (highly suspect finding)
- Monotonous, uniform hepatocytic cell population with subtle malignant features
- Pseudoacinar formation in cell clusters
- Nuclear-to-cytoplasmic ratio higher than in normal hepatocytes (>1/3)
- Macroeosinophilic nucleoli
- Reduced number of binucleated cells
- Background free of bile duct epithelial cells
- Reticulin stain demonstrates a loss of the normal 1 to 2 cells thick hepatic plate architecture
- Iron stain fails to stain tumor cells in cases of hemochromatosis
- α-fetoprotein is helpful if positive but often is not
- Novel markers, such as glypican-3, glutamine synthetase and heat shock protein 70, are helpful if two out of three show positivity
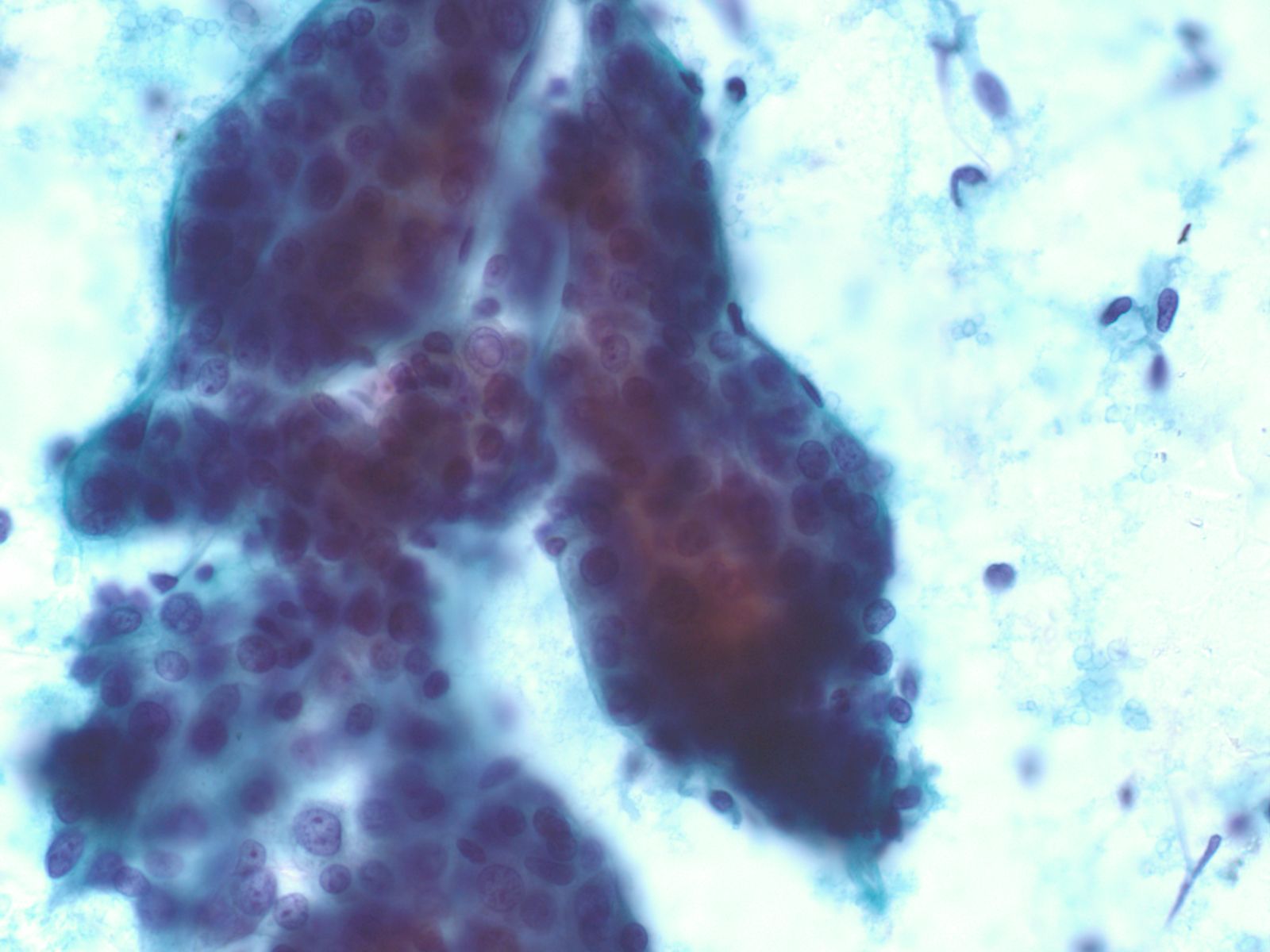 |
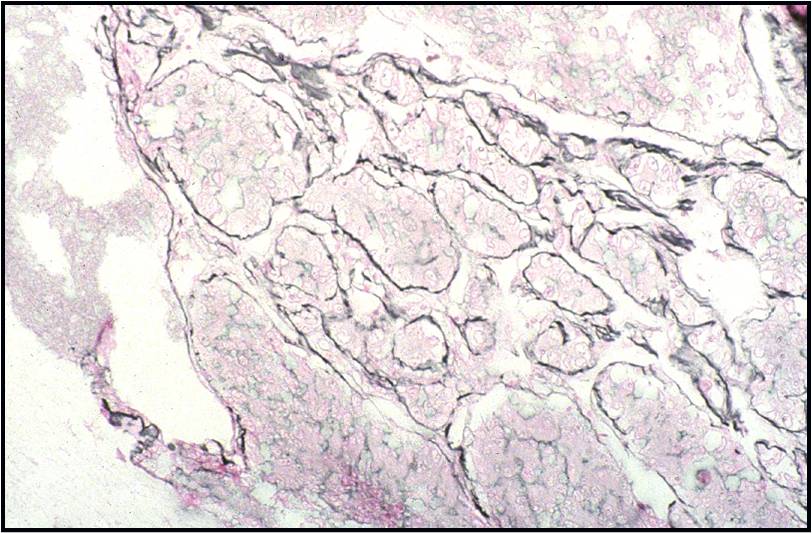 |
|
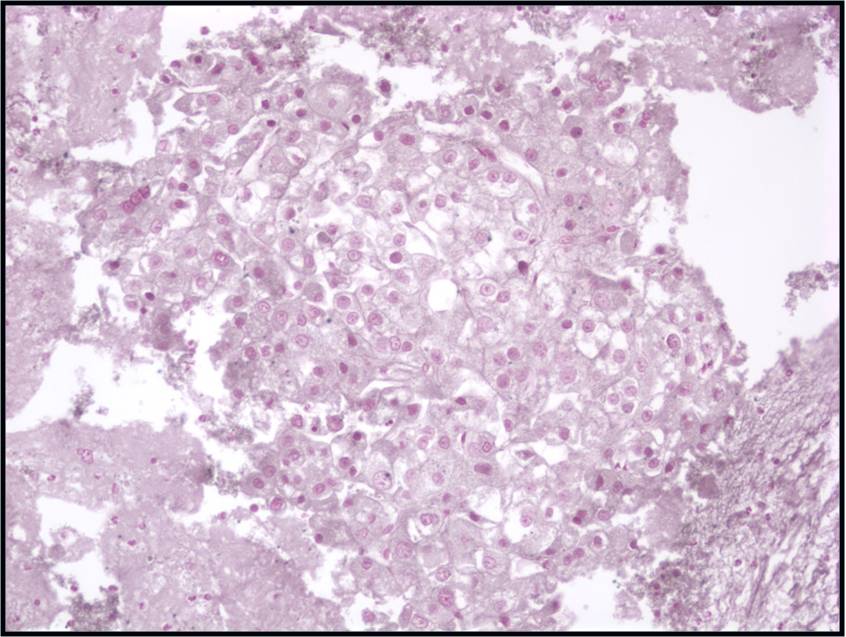 |
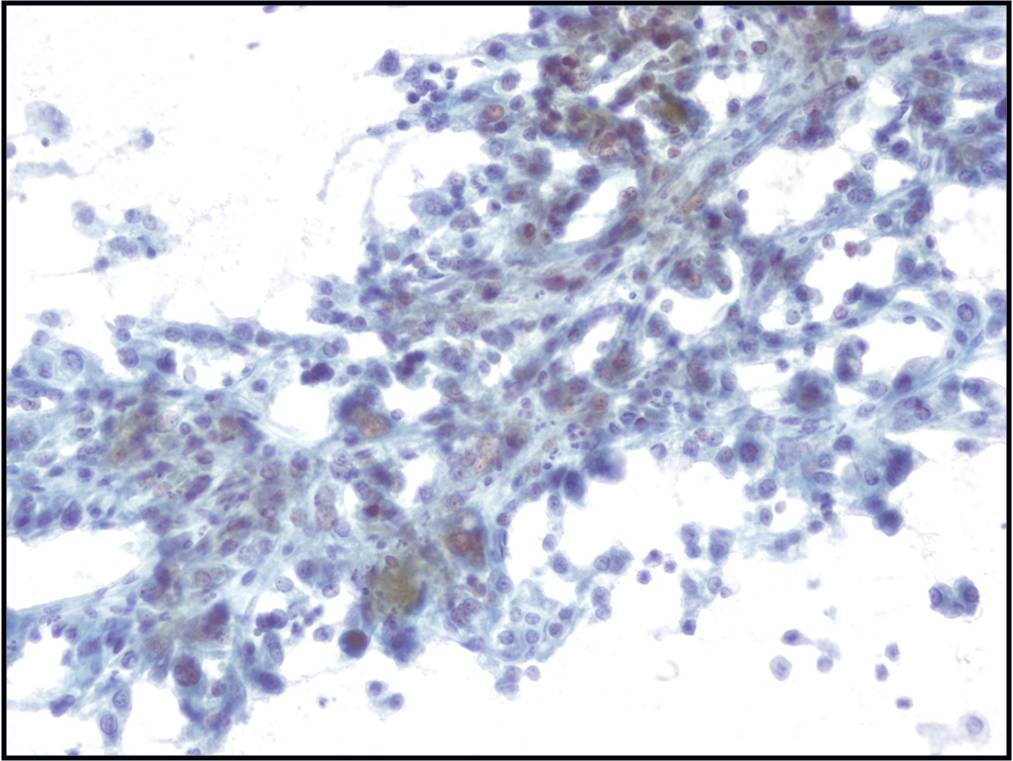 |
|
Moderately to Poorly Differentiated Hepatocellular Carcinoma –N12-6639, NC13-322 and N11-12944
- Low power smear pattern generally resembles that seen in well-differentiated tumors; however, there is a dyshesive tendency in poorly differentiated tumors
- Peripheral endothelium is virtually pathognomonic
- Transgressing vessels are suggestive, but cannot distinguish hepatocellular from renal cell carcinoma
- Presence of intracytoplasmic bile is pathognomonic
- Polygonal cells with central nuclei and prominent nucleoli with visible, granular to clear cytoplasm in moderately differentiated tumors; scant to no cytoplasm with greater degree of pleomorphism and mitotic activity in poorly differentiated tumors
- Immunophenotype: low-molecular-weight CK (Cam 5.2), polyclonal carcinoembryonic antigen and CD10 (canalicular), and HepPar-1 and TTF-1 positive; α-fetoprotein variable; high-molecular-weight CK (AE1) negative
 |
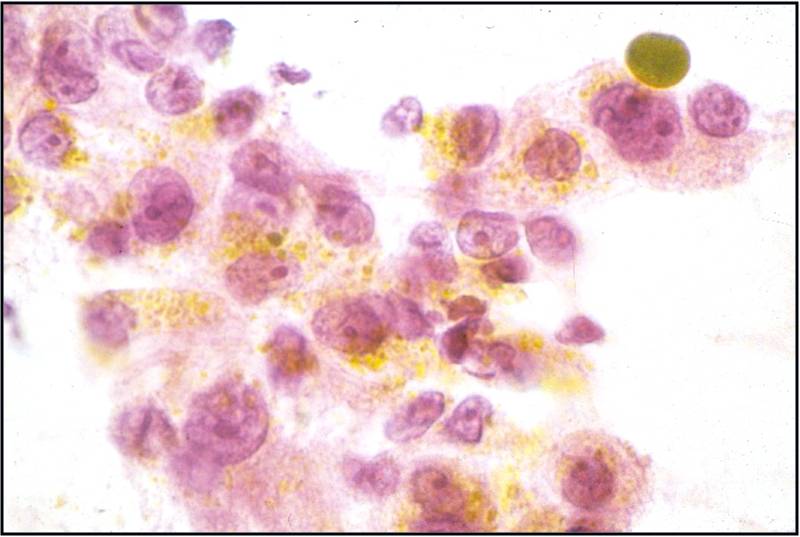 |
|
Metastatic colonic Adenocarcinoma -- N12-85
- Cigar-shaped, often palisaded nuclei
- Variably prominent nucleoli, no macroeosinophilic nucleoli
- Dirty necrosis in the background (key identification point)
- Immunohistochemistry: CK20 positive, CK7 and CK19 negative, carcinoembryonic antigen positive
 |
||
Cytology service page
Warning: Display title "1-11 Pancreas FNA: M Pitman MD, J Misdraji MD, M Nutter CT" overrides earlier display title "1-10 Liver Cytology: M Pitman MD, J Misdraji MD, Marilyn Nutter CT".
The clinical evaluation of patients who present with a biliary stricture, pancreatic cyst or solid mass begins when the patient comes to medical attention due to symptoms, or when a lesion is discovered incidentally during work-up for another reason. Managing these patients is challenging due to the wide variety of options for treatment ranging from observation to surgery. Carcinoma is a clinical concern for patients with newly diagnosed late onset diabetes mellitus or unexplained acute pancreatitis in an older individual.1 Development of jaundice, pruritus or cholestasis not explained by underlying liver or gallbladder disease or choledocholithiasis should initiate an evaluation for neoplasia of the hepaticobiliary tract. 2,3 Abdominal pain that radiates to the back, anorexia, weight loss, steatorrhea, and abnormal liver enzymes are also common presenting symptoms of pancreaticobiliary disease.
There are many imaging modalities that can be used in evaluating the pancreas and biliary tract (Table 1). The general standard “pancreas protocol” begins with a computed tomography (CT) scan, usually multiphase or multidetector CT (MDCT) with contrast. Magnetic resonance imaging (MRI) techniques including MR cholangiopancreatography (MRCP) and MR angiography (MRA) are used in selected cases such as small lesions, unclear solid versus cystic composition or in search of a connection to the main pancreatic duct in suspected branch-duct intraductal papillary mucinous neoplasms(IPMN) .4,5 Endoscopic retrograde cholangiopancreatography (ERCP) is most useful clinically for providing relief of obstructive jaundice by either plastic or metal biliary stent placement. Brush cytology from biliary strictures or, to a lesser extent, pancreatic duct strictures, is utilized to sample duct strictures. It is only rarely used as a primary tool for establishing the diagnosis of pancreatic adenocarcinoma or in the work-up of pancreatic cysts as these lesions are best evaluated with EUS-FNA.
For pancreatic cysts, the most important clinical determination is whether a pancreatic cyst is mucinous or non-mucinous on the one hand, and benign or malignant (high-grade or high-risk cyst) on the other.6 This distinction by imaging alone is quite challenging, but imaging classification systems have been proposed.7 Most patients with inflammatory pseudocysts can be managed medically and serous cysts are benign neoplasms that do not warrant surgical resection unless the patient is symptomatic or the cyst is large (> 4cm)8. Management guidelines have been proposed for suspected mucinous cysts.9 These guidelines base management primarily on clinical and imaging findings with conservative management of small cysts (< 3 cm) occurring in patients without high-risk clinical or imaging findings. Patients with high-risk imaging features or ≥suspicious cytology are triaged to surgery. Patients with worrisome imaging features should be evaluated by endoscopic ultrasound (EUS) and fine-needle aspiration (FNA) biopsy for further assessment of high-risk features.
For solid masses EUS is used for preoperative staging in patients with suspected pancreatic or biliary tract carcinoma. EUS is particularly useful when CT or MR findings are equivocal, in CT-resectable disease to screen for subtle vascular involvement (especially in borderline surgical candidates), or when unresectable disease is suspected and a tissue diagnosis is needed.10 EUS is better than CT and MR in establishing the presence of vascular invasion, and for detecting small masses, evaluating for tumor involvement of the superior mesenteric and portal veins and for detecting lymph node metastases. 4,11,12
A key advantage of EUS is that EUS-FNA can be performed as an integral part of EUS examination. This allows definitive tissue acquisition and rapid diagnosis. When EUS suggests resectability, EUS-guided biopsy may not be necessary prior to definitive resection. EUS-FNA can confirm the presence of metastatic disease in image detected lymphadenopathy. Patients considered candidates for preoperative neoadjuvant therapy require a definitive tissue diagnosis. Aspiration of pancreatic cysts provides fluid for biochemical, cytological and molecular analysis that help to determine the nature of the cyst and if the patient requires surgical or conservative management.
All specimens submitted to the pathology laboratory are accompanied by a specimen requisition form. This form is a means of communication, not only to the pathologist interpreting the specimen, but also to the laboratory personnel who process the specimen. Often tissue management protocols are in place for certain specimen types and clinical diseases, so this information must be clearly delineated on the requisition form. Additional helpful information specific to the pancreaticobiliary tract includes precise site of the lesion being sampled, the tissue traversed during FNA (stomach or duodenum), the solid or cystic nature of the lesion, the presence or absence of bile duct or pancreatic duct stricture, the size of the lesion and the presence of high-risk or worrisome features. For pancreatic cysts, the configuration of the cyst is vital, e.g. borders, loculations, wall thickness, mural nodules or masses, etc. Clinical suspicion of neuroendocrine tumor or lymphoma should be indicated as tissue for the proper evaluation of these neoplasms requires special handling.
1. Guidelines for the management of patients with pancreatic cancer periampullary and ampullary carcinomas. Gut. Jun 2005;54 Suppl 5:v1-16.
2. Baron TH, Mallery JS, Hirota WK, et al. The role of endoscopy in the evaluation and treatment of patients with pancreaticobiliary malignancy. Gastrointest Endosc. Nov 2003;58(5):643-649.
3. Patel AH, Harnois DM, Klee GG, LaRusso NF, Gores GJ. The utility of CA 19-9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am J Gastroenterol. Jan 2000;95(1):204-207.
4. Midwinter MJ, Beveridge CJ, Wilsdon JB, Bennett MK, Baudouin CJ, Charnley RM. Correlation between spiral computed tomography, endoscopic ultrasonography and findings at operation in pancreatic and ampullary tumours. Br J Surg. Feb 1999;86(2):189-193.
5. Megibow A, Zhou X, Rortterdam H. Pancreatic adenocarcinoma: CT versus MR imaging in the evaluation of resectabiilty--report of the radiology diagnostic oncology group. Radiology. 1995(195):327-332.
6. Pitman MB, Brugge WR, Warshaw AL. The value of cyst fluid analysis in the pre-operative evaluation of pancreatic cysts. J Gastrointest Oncol. Dec 2011;2(4):195-198.
7. Sahani DV, Kadavigere R, Saokar A, Fernandez-del Castillo C, Brugge WR, Hahn PF. Cystic pancreatic lesions: a simple imaging-based classification system for guiding management. Radiographics. Nov-Dec 2005;25(6):1471-1484.
8. Tseng JF, Warshaw AL, Sahani DV, Lauwers GY, Rattner DW, Fernandez-del Castillo C. Serous cystadenoma of the pancreas: tumor growth rates and recommendations for treatment. Ann Surg. 2005;242(3):413-419; discussion 419-421.
9. Tanaka M, Fernandez-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. May-Jun 2012;12(3):183-197.
10. Palazzo L, Roseau G, Gayet B, et al. Endoscopic ultrasonography in the diagnosis and staging of pancreatic adenocarcinoma. Results of a prospective study with comparison to ultrasonography and CT scan. Endoscopy. 1993;25:143-150.
11. Howard TJ, Chin AC, Streib EW, Kopecky KK, Wiebke EA. Value of helical computed tomography, angiography, and endoscopic ultrasound in determining resectability of periampullary carcinoma. Am J Surg. Sep 1997;174(3):237-241.
12. Legmann P, Vignaux O, Dousset B, et al. Pancreatic tumors: comparison of dual-phase helical CT and endoscopic sonography. AJR. Am. J. Roentgenol. May 1998;170(5):1315-1322.
Techniques for Cytologic Sampling of Pancreatic and Bile Duct Lesions1
Major developments in the diagnosis and management of patients with pancreaticobiliary diseases have stemmed from improvements in sampling of the pancreas and biliary system, principally through the advent of EUS-FNA 2. EUS-FNA is now the procedure of choice for establishing a diagnosis of pancreatic malignancy, and the recent development of core needles that can be used with EUS will further improve diagnostic accuracy.
Endoscopic retrograde cholangiopancreatography is the traditional and simplest method of obtaining a cytology specimen of a biliary stricture3. Brush cytology has a much better yield than bile aspiration alone, and brushing cytology provides a more accurate diagnosis than directed focal biopsy because the entire surface area of the stricture is sampled by the brush4. Tissue can be smeared on a slide or released from the brush in preservative solutions for liquid-based processing. 5 Biopsies are often used to supplement brush cytology. When comparing brushing, standard forceps and mini-forceps methods of sampling, mini-forceps biopsy provided significantly better sensitivity and overall accuracy compared with cytology brushing and standard forceps biopsy. 6 Needle biopsy of biliary strictures and masses can also be performed with fluoroscopic guidance 7. Endoscopic cholangioscopy is another technique performed using a dedicated, small diameter endoscope that is placed through the instrument channel of a duodenoscope. Finally, EUS-FNA of a bile duct mass may also be used as a technique to establish the diagnosis of malignancy.
EUS-FNA of pancreatic masses requires linear endosonographic instruments 8. The appropriate gauge EUS needle should be selected for the procedure based on the vascularity of the target lesion, the difficulty in accessing the lesion, and the type of tissue needed for a diagnosis. Simple aspiration needles (usually 22- or 25-gauge) are used in the vast majority of biopsies and provide similar cytologic yield 9. Highly vascular lesions such as neuroendocrine tumors and metastatic renal cell carcinoma as well as lesions of the uncinate process should be aspirated with a 25-gauge needle. Smaller gauge needles are easier to use and generally safer. Mucinous cysts are aspirated with 22-gauge needles because of the high viscosity of the cyst fluid. Core biopsy and Trucut needles (19-gauge) are used for fibrotic and stromal rich lesions such as autoimmune pancreatitis. Small gauge core biopsy needles have recently been made available and are often used when standard aspiration techniques do not provide a diagnostic tissue. FNA of cystic lesions one passage of the needle should be used to evaluate a cyst and high suction will aid in the rapid emptying of the cyst. Mural nodules or adjacent masses should be targeted or aspirated separately.
Whenever possible, rapid on site evaluation (ROSE) of cytology should be used in FNAs of solid masses since it reduces the frequency of false-negative FNAs, particularly in the evaluation of pancreatic masses 10. Without ROSE, approximately, 5 passes of a pancreatic mass are needed to ensure adequate sampling. ROSE is not advised for FNAs of cysts unless there is a solid component that provides tissue for direct smears. Aspirated cyst fluid should be carefully triaged for cytology, biochemical testing (CEA and amylase), and molecular testing.
1. Brugge W, Dewitt J, Klapman JB, et al. Techniques for cytologic sampling of pancreatic and bile duct lesions. Diagn Cytopathol. Feb 19 2014.
2. Yoon WJ, Brugge WR. Endoscopic evaluation of bile duct strictures. Gastrointest Endosc Clin N Am. Apr 2013;23(2):277-293.
3. Ferrari AP, Lichtenstein DR, Slivka A, Chang C, Carr-Locke DL. Brush cytology during ERCP for the diagnosis of biliary and pancreatic malignancies. Gastrointest Endosc. 1994;40:140-145.
4. Ung KA, Ljung A, Wagermark J, et al. Brush cytology is superior to biopsies obtained by a new device in bile duct strictures. Hepatogastroenterology. Apr-May 2007;54(75):664-668.
5. Vadmal MS, Byrne-Semmelmeier S, Smilari TF, Hajdu SI. Biliary tract brush cytology. Acta Cytol. Jul-Aug 2000;44(4):533-538.
6. Draganov PV, Chauhan S, Wagh MS, et al. Diagnostic accuracy of conventional and cholangioscopy-guided sampling of indeterminate biliary lesions at the time of ERCP: a prospective, long-term follow-up study. Gastrointest Endosc. Feb 2012;75(2):347-353.
7. Howell DA, Beveridge RP, Bosco J, Jones M. Endoscopic needle aspiration biopsy at ERCP in the diagnosis of biliary strictures. Gastrointest Endosc. Sep-Oct 1992;38(5):531-535.
8. Vilmann P, Saftoiu A. Endoscopic ultrasound-guided fine needle aspiration biopsy: equipment and technique. J Gastroenterol Hepatol. Nov 2006;21(11):1646-1655.
9. Lee JH, Stewart J, Ross WA, Anandasabapathy S, Xiao L, Staerkel G. Blinded prospective comparison of the performance of 22-gauge and 25-gauge needles in endoscopic ultrasound-guided fine needle aspiration of the pancreas and peri-pancreatic lesions. Dig. Dis. Sci. Oct 2009;54(10):2274-2281.
10. Collins BT, Murad FM, Wang JF, Bernadt CT. Rapid on-site evaluation for endoscopic ultrasound-guided fine-needle biopsy of the pancreas decreases the incidence of repeat biopsy procedures. Cancer Cytopathol. Sep 2013;121(9):518-524.
Standardized Terminology and Nomenclature 1
The proposed terminology scheme has six categories (Table 2). New and somewhat controversial is the category “Neoplastic” that is divided into clearly “benign” neoplasms and “other” neoplasms. Using a standardized terminology and nomenclature system provides intra- and interdepartmental guidance for diagnosis and attempts to correlate the diagnosis with our current understanding of the lesion's biological behavior and management recommendations. Interpretation categories do not have to be used, but some pathology laboratory information systems require them, and such categorization may aide in clinical and translational research.
Category I. Non-Diagnostic
A non-diagnostic cytology specimen is one that provides no diagnostic or useful information about the solid or cystic lesion sampled; for example, an acellular aspirate of a cyst without evidence of a mucinous etiology by cytology and ancillary testing.1 Cellular atypia precludes a non-diagnostic report regardless of the cellularity. The clinical and imaging context should be taken into consideration when assessing whether a sample is adequate. The absence of "epithelial cells" in the sample does not necessarily make a specimen non-diagnostic. A pseudocyst, for example, lacks an epithelial cyst lining by definition, and thick colloid-like mucin without epithelial cells may be aspirated from mucinous cysts, but this finding or an elevated CEA level in the cyst fluid, is sufficient to support an interpretation of a neoplastic mucinous cyst. 2-4
Category II. Negative (for malignancy)
A negative cytology sample is one that contains adequate cellular and/or extracellular tissue to evaluate or define a lesion that is identified on imaging. When using the negative category one should give a specific diagnosis when practical; for example, pancreatitis (acute, chronic and autoimmune), pseudocyst and lymphoepithelial cyst.1 Benign pancreaticobiliary tissue in the setting of vague fullness and no discrete mass also qualifies as a negative interpretation. A negative interpretation with a descriptive diagnosis implies that the sample is adequately cellular and that no cytological atypia is present. A “negative” report with a descriptive diagnosis such as "mucinous debris of uncertain origin (lesional versus gastrointestinal contamination)" is reasonable.
Category III. Atypical
The atypical category is appropriate when cells display cytoplasmic, nuclear, or architectural features inconsistent with normal or reactive cellular changes, and that are insufficient to classify the cells as a neoplasm or suspicious for a high-grade malignancy. The category is heterogeneous and includes cases with reactive changes, low cellularity specimens, premalignant changes (dysplasia) and cases assigned to this category due to observer caution in diagnosis. In addition, the cytological findings may be suggestive but not diagnostic of a low-grade neoplasm due to insufficient tissue for confirmation of a specific diagnosis. Brushing cytology yielding atypical biliary epithelium remains in this category since premalignant lesions of the biliary tract have not been as well defined with correlative management algorithms.
Category IV. Neoplastic
IVA. Neoplastic: Benign
This interpretation category connotes the presence of a cytological specimen sufficiently cellular and representative, with or without the context of clinical, imaging, and ancillary studies, to be diagnostic of a benign neoplasm. The most commonly encountered benign neoplasm of the pancreas is serous cystadenoma. Other benign neoplasms of the pancreas such as cystic teratoma and schwannoma are extremely rare and are also placed in this category.
IVB. Neoplastic: Other
This interpretation category defines a neoplasm that is either premalignant such as IPMN or mucinous cystic neoplasm (MCN) with low, intermediate or high-grade dysplasia using cytological criteria, or a low-grade malignant neoplasm such as well-differentiated pancreatic neuroendocrine tumors (PanNET) or solid-pseudopapillary neoplasms (SPN).
This category represents the most controversial aspect of this terminology proposal. The rationale for this proposed category relates the desire to standardize and correlate the cytological nomenclature with the 2010 WHO terminology classification that maintains the nomenclature for both PanNET and SPN as "neoplasms" rather than carcinomas, and to take into consideration the increasingly conservative management approaches for many of the lesions.
These "other" neoplasms are either pre-invasive, premalignant neoplasms (IPMN and MCN with low, intermediate or high-grade dysplasia) or demonstrate low-grade malignant behavior (PanNET and SPN), and, as such, warrant distinction from aggressive, high-grade malignancies (most notably ductal adenocarcinoma). All of the tumors in this category are clearly neoplastic, and even though some are low-grade malignant, the heading “Neoplastic: Other” is an accurate and reasonable generic term that accurately reflects the pre-operative cytological terminology and does not define the neoplasm as benign or malignant. The cytological categories of “atypical” and “suspicious for malignancy” connote an indeterminate interpretation and do not relate the detection of a neoplasm, which could lead to unnecessary repeat biopsy.
The cytological interpretation of PanNET indicates a well-differentiated neoplasm. Although it is now widely accepted that well-differentiated PanNETs all have malignant potential5, many PanNET are very slow growing, and even curable if caught at an early stage, and some are detected incidentally in asymptomatic, elderly patients. To distinguish PanNETs from highly aggressive malignant neoplasms and to offer management flexibility in elderly patients with small, asymptomatic tumors where the risk to benefit ratio of surgery is high compared to conservative management, PanNETs are placed in this category rather than the malignant category. Convincing a patient that conservative management of their incidental 1 cm PanNET is the best option for them is virtually impossible when diagnosed by cytology as malignant.
Solid-pseudopapillary neoplasm is a low-grade malignancy but with a small local recurrence rate and low metastatic potential. 6 For these reasons coupled with the fact that the tumor is called a "neoplasm" and not carcinoma, it is included in this Neoplastic: Other category.
The two primary neoplastic mucinous cysts of the pancreas include IPMN and MCN. Premalignant cysts are lined by low, intermediate or high-grade dysplasia; malignant counterparts require invasive carcinoma. Atypia less than overtly malignant is included in this category of Neoplastic: Other. Distinguishing the atypia in these cysts is challenging using a 4 tiered system, and it is not always possible to distinguish high-grade dysplasia and carcinoma, or intermediate-grade dysplasia and high-grade dysplasia. A two-tiered system of low-grade (low-grade and intermediate-grade dysplasia) and high-grade (high-grade dysplasia or adenocarcinoma) epithelial atypia provides the best information for clinical management.7-10 A diagnosis of adenocarcinoma (positive or malignant category) requires unequivocal features of adenocarcinoma.
Category IV. Suspicious (for Malignancy)
A specimen is suspicious for malignancy when some but an insufficient number of the typical features of a specific malignant neoplasm, mainly pancreatic adenocarcinoma, are present. The cytological features raise a strong suspicion for malignancy, but the findings are qualitatively and/or quantitatively insufficient for a conclusive diagnosis, or tissue is not present for ancillary studies to define a specific neoplasm. The morphologic features must be sufficiently atypical that malignancy is considered more probable than not. 1
This category of “suspicious for malignancy” generally refers to pancreatic adenocarcinoma since most malignancies in the pancreas are ductal adenocarcinoma, but this category is used for all high-grade, aggressive malignancies. The suspicious category is an appropriate classification for aspirates that produce a "solid-cellular" clearly neoplastic epithelial proliferation which includes PanNET, acinar cell carcinoma, pancreatoblastoma and SPN in the differential diagnosis, but which has insufficient tissue for confirmatory ancillary studies necessary to make a specific diagnosis. This category has a very high positive predictive value for malignancy.11-13 The risk of malignancy for brushing specimens designated “suspicious for malignancy” is approximately 80% and 96%.14
Category VI. Positive or Malignant
This category includes a group of neoplasms that unequivocally display malignant cytological characteristics including pancreatic ductal adenocarcinoma and its variants, cholangiocarcinoma, acinar cell carcinoma, high-grade neuroendocrine carcinoma (small cell and large cell), pancreatoblastoma, lymphomas, sarcomas and metastases to the pancreas.
Since 9 of 10 malignancies in the pancreas are conventional ductal adenocarcinoma, the "positive" or "malignant" category is often related to this neoplasm. The specificity of a positive or malignant interpretation for both pancreatic FNA and biliary brushing is very high, >90-95% in most studies 11,15-22. Relying on strict criteria contributes to this high specificity at the expense of sensitivity. Rapid on site evaluation of solid mass lesion FNAs contributes to diagnostic yield 23-25.
1. Pitman MB, Centeno BA, Ali SZ, et al. Standardized terminology and nomenclature for pancreatobiliary cytology: The Papanicolaou Society of Cytopathology guidelines. Diagn Cytopathol. Feb 19 2014.
2. Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. May 2004;126(5):1330-1336.
3. Cizginer S, Turner B, Bilge AR, Karaca C, Pitman MB, Brugge WR. Cyst Fluid Carcinoembryonic Antigen Is an Accurate Diagnostic Marker of Pancreatic Mucinous Cysts. Pancreas. Jul 14 2011;40(7):1024-1028.
4. van der Waaij LA, van Dullemen HM, Porte RJ. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: a pooled analysis. Gastrointest Endosc. Sep 2005;62(3):383-389.
5. Klimstra DS. Pathology reporting of neuroendocrine tumors: essential elements for accurate diagnosis, classification, and staging. Semin Oncol. Feb 2013;40(1):23-36.
6. Kloppel G, Hruban R, Klimstra D, et al. Solid-pseudopapillary neoplasm of the pancreas. In: Bosman F, Carneiro F, Hruban R, Theise N, eds. 4th ed. Sterling: Stylus Publishing, LLC; 2010:327-330.
7. Pitman MB, Centeno BA, Daglilar ES, Brugge WR, Mino-Kenudson M. Cytological criteria of high-grade epithelial atypia in the cyst fluid of pancreatic intraductal papillary mucinous neoplasms. Cancer Cytopathol. Jan 2014;122(1):40-47.
8. Pitman MB, Centeno BA, Genevay M, Fonseca R, Mino-Kenudson M. Grading epithelial atypia in endoscopic ultrasound-guided fine-needle aspiration of intraductal papillary mucinous neoplasms: An international interobserver concordance study. Cancer Cytopathol. Dec 2013;121(12):729-736.
9. Pitman MB, Genevay M, Yaeger K, et al. High-grade atypical epithelial cells in pancreatic mucinous cysts are a more accurate predictor of malignancy than "positive" cytology. Cancer Cytopathol. Oct 7 2010;118:434-440.
10. Pitman MB, Lewandrowski K, Shen J, Sahani D, Brugge W, Fernandez-del Castillo C. Pancreatic cysts: preoperative diagnosis and clinical management. Cancer Cytopathol. Feb 25 2010;118(1):1-13.
11. Bhutani MS, Hawes RH, Baron PL, Sanders CA, van VA, Osborne. Endoscopic ultrasound guided fine needle aspiration of malignant pancreatic. Endoscopy. 1997;29(9):854-858.
12. Faigel DO, Ginsberg GG, Bentz JS, Gupta PK, Smith DB, Kochman ML. Endoscopic ultrasound-guided real-time fine-needle aspiration biopsy of the pancreas in cancer patients with pancreatic lesions. J Clin Oncol. 1997;15(4):1439-1443.
13. Eloubeidi MA, Jhala D, Chhieng DC, et al. Yield of endoscopic ultrasound-guided fine-needle aspiration biopsy in patients with suspected pancreatic carcinoma. Cancer. Oct 25 2003;99(5):285-292.
14. Layfield LJ, Schmidt RL, Hirschowitz SL, Olson MT, Ali SZ, Dodd LL. Significance of the diagnostic categories "atypical" and "suspicious for malignancy" in the cytologic diagnosis of solid pancreatic masses. Diagn Cytopathol. Feb 28 2014.
15. Layfield LJ, Wax TD, Lee JG, al e. Accuracy and morphologic aspects of pancreatic and biliary duct brushings. Acta Cytol. 1995;39:11-18.
16. Turner BG, Cizginer S, Agarwal D, Yang J, Pitman MB, Brugge WR. Diagnosis of pancreatic neoplasia with EUS and FNA: a report of accuracy. Gastrointest Endosc. Jan 2010;71(1):91-98.
17. Ylagan LR, Edmundowicz S, Kasal K, Walsh D, Lu DW. Endoscopic ultrasound guided fine-needle aspiration cytology of pancreatic carcinoma: a 3-year experience and review of the literature. Cancer. Dec 25 2002;96(6):362-369.
18. Ylagan LR, Liu LH, Maluf HM. Endoscopic bile duct brushing of malignant pancreatic biliary strictures: retrospective study with comparison of conventional smear and ThinPrep techniques. Diagn Cytopathol. Apr 2003;28(4):196-204.
19. Eloubeidi M, Chen V, Eltoum I, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy of patients with suspected pancreatic cancer: diagnostic accuracy and acute and 30-day complications. Am J Gastroenterol. 2003;98:2663-2668.
20. Eloubeidi MA, Tamhane A. EUS-guided FNA of solid pancreatic masses: a learning curve with 300 consecutive procedures. Gastrointest Endosc. May 2005;61(6):700-708.
21. Adamsen S, Olsen M, Jendresen MB, Holck S, Glenthoj A. Endobiliary brush biopsy: Intra- and interobserver variation in cytological evaluation of brushings from bile duct strictures. Scand J Gastroenterol. May 2006;41(5):597-603.
22. Volmar KE, Vollmer RT, Routbort MJ, Creager AJ. Pancreatic and bile duct brushing cytology in 1000 cases: review of findings and comparison of preparation methods. Cancer. Aug 25 2006;108(4):231-238.
23. Hebert-Magee S, Bae S, Varadarajulu S, et al. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: a meta-analysis. Cytopathology. Jun 2013;24(3):159-171.
24. Klapman JB, Logrono r, Dye CE, Waxman I. Clinical impact of on-site cytopathology interpretation on endoscopic ultrasound-guided fine needle aspiration. Am J Gastroenterol. 2003;98(6):1289-1294.
25. da Cunha Santos G, Ko HM, Saieg MA, Geddie WR. "The petals and thorns" of ROSE (rapid on-site evaluation). Cancer Cytopathol. Jul 3 2012.
Basic cytomorphology
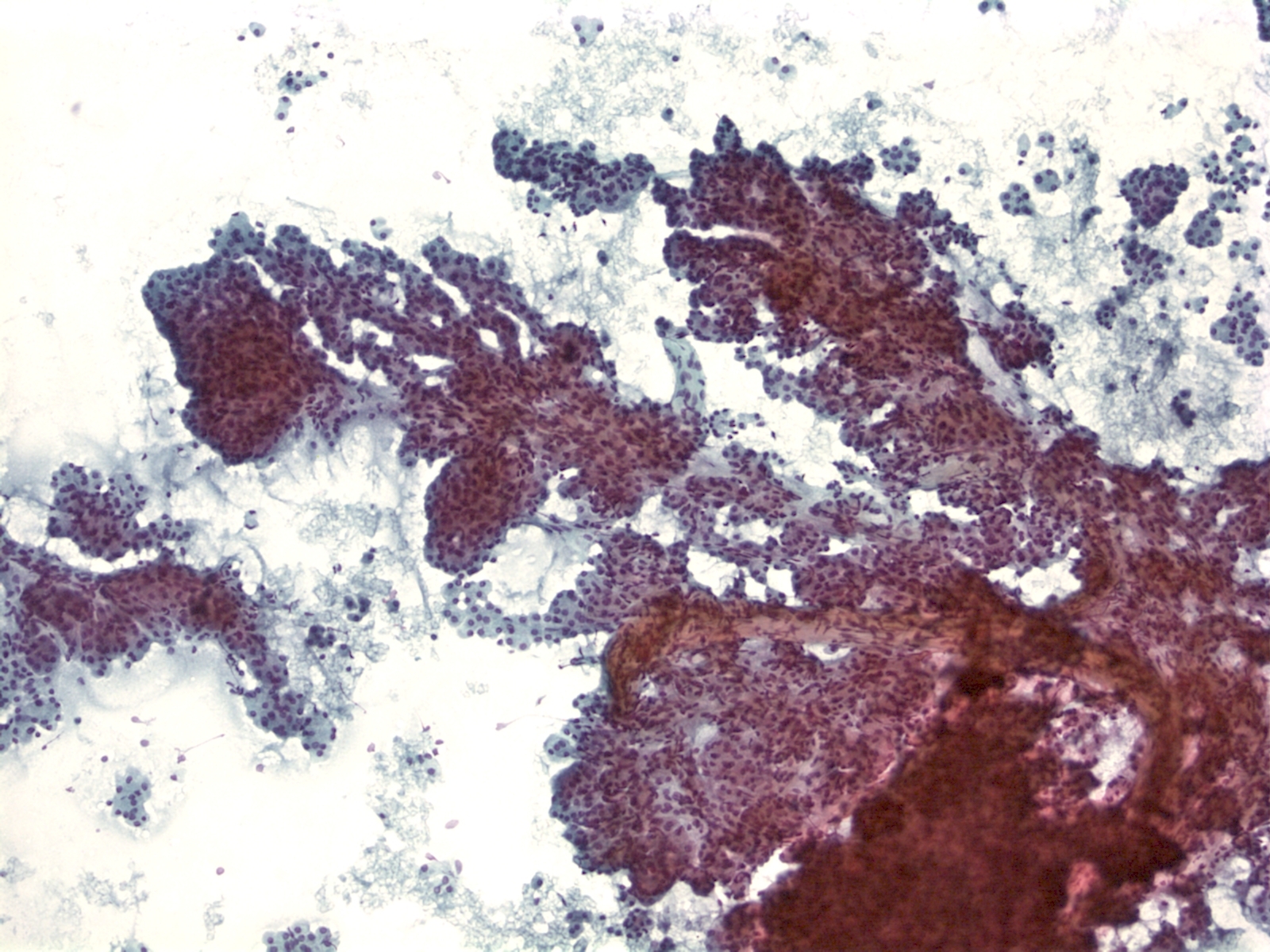 |
||
Benign Pancreas – PPANC4-04 Key Cytological Features of benign acinar cells:
- Cohesive, grape-like aggregates singly and attached to fibrovascular stroma
- Scattered stripped naked nuclei
- Basally located round nucleus
- Finely granular chromatin
- Small nucleolus; larger in reactive acinar cells
- Abundant granular cytoplasm
- Indistinct cell borders in clusters
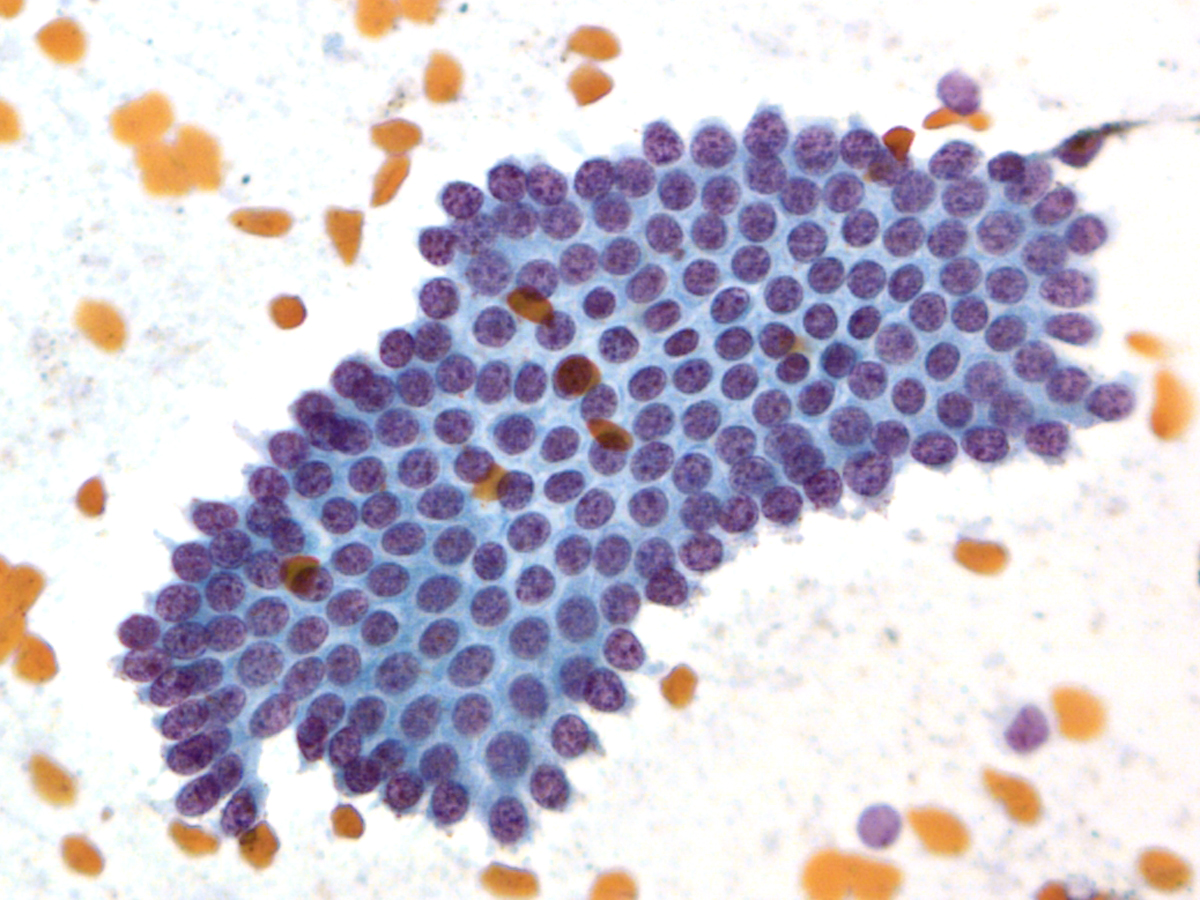 |
||
Key Cytological Features of Ductal Epithelium:
- Flat, cohesive sheets with even nuclear spacing within sheets
- Uniform, on-edge "picket-fence" arrangements
- Round to oval nuclei with fine, even chromatin
- Inconspicuous nucleoli
- Non-mucinous cytoplasm
 |
||
EUS Contaminants
Duodenal Epithelium– MN05-N08394
- Flat and cohesive monolayered sheet with a honeycomb pattern; occasionally papillary groups (intact villi), smaller groups and single cells
- Non-mucinous glandular cells with brush border
- Sporadically placed goblet cells appearing as “fried eggs” with a sheet
- Lymphocytes (“sesame seeds”) in the epithelium
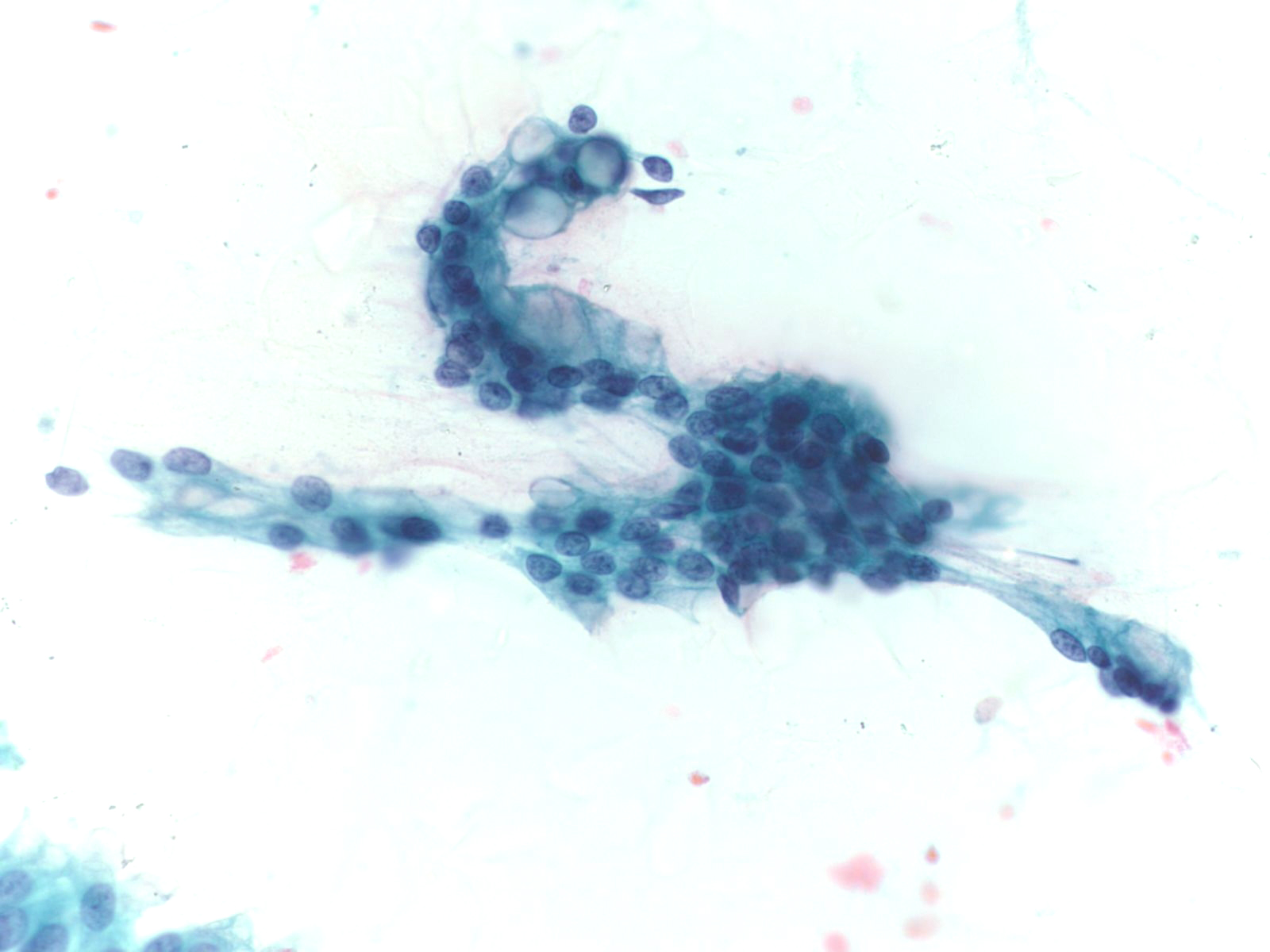 |
||
Gastric Epithelium– N13-6431
- Small sheets, strips and occasionally single cells and gastric pits
- Visible mucin in foveolar cells, often apical mucin cups
- Grooved naked nuclei, typically floating in extracellular mucin
Ductal Adenocarcinoma
- Incidence: 11 per 100,000 ; 4th leading cause of cancer death in men and women
- Age, Gender: Peak incidence 7th to 8th decade; M>F by 30%
- Prognosis and Therapy: 5-year survival rate is 3-4%. Surgical resection is the treatment of choice.
- Radiological Features: Hypodense mass with a poorly defined periphery and often a “double duct sign” from dilatation of both the pancreatic and bile ducts.
Key Cytologic Features: Ductal Adenocarcinoma
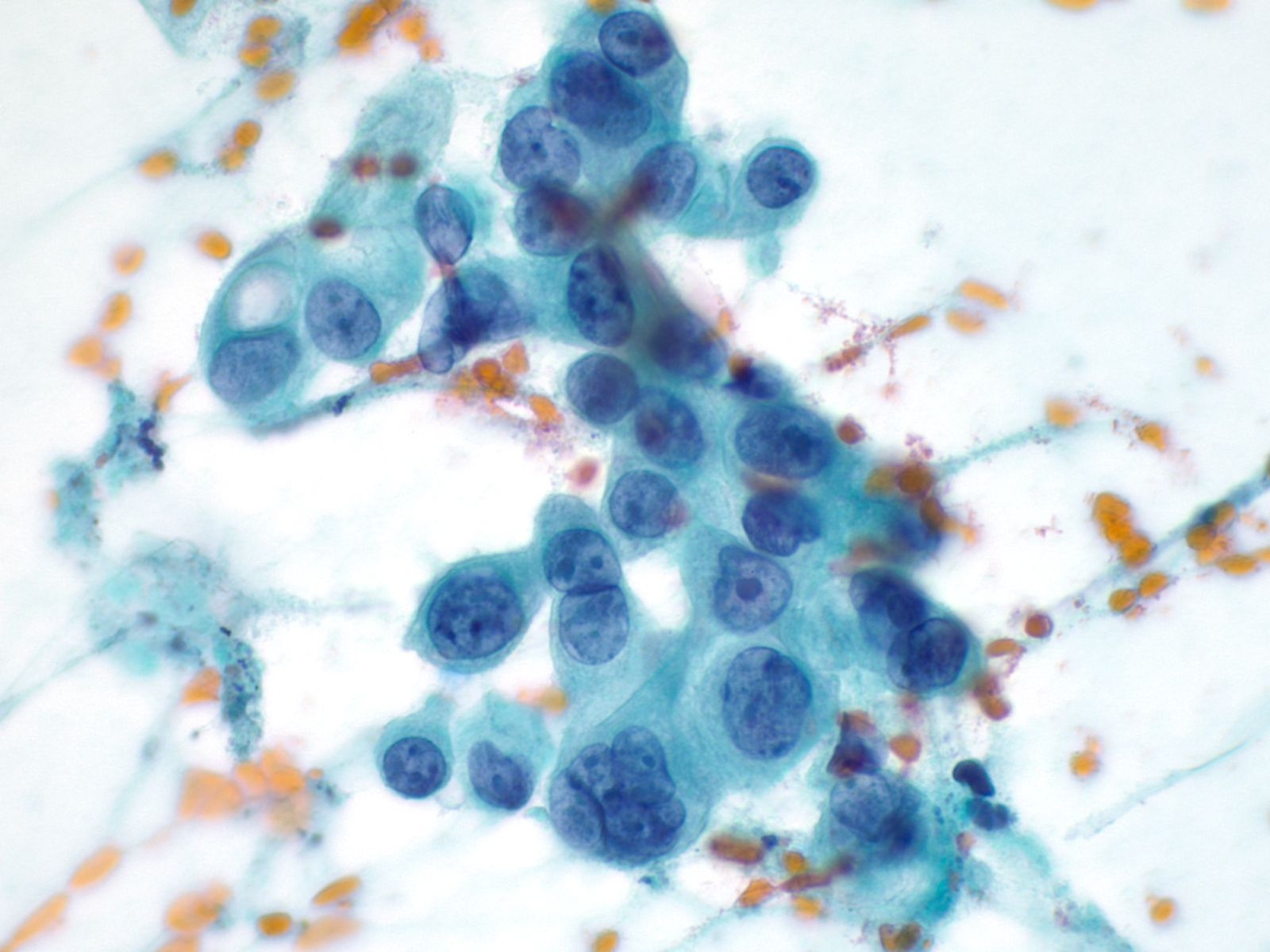 |
||
High Grade Adenocarcinoma – PPAN4-06
- Glandular smear pattern with ductal cells in variable amounts
- Three-dimensional groups with nuclear crowding, overlap and loss of polarity
- Obvious nuclear membrane irregularities, hyperchromasia, coarse chromatin and prominent nucleoli
- Mitosis, necrosis and dyscohesion with single intact malignant cells
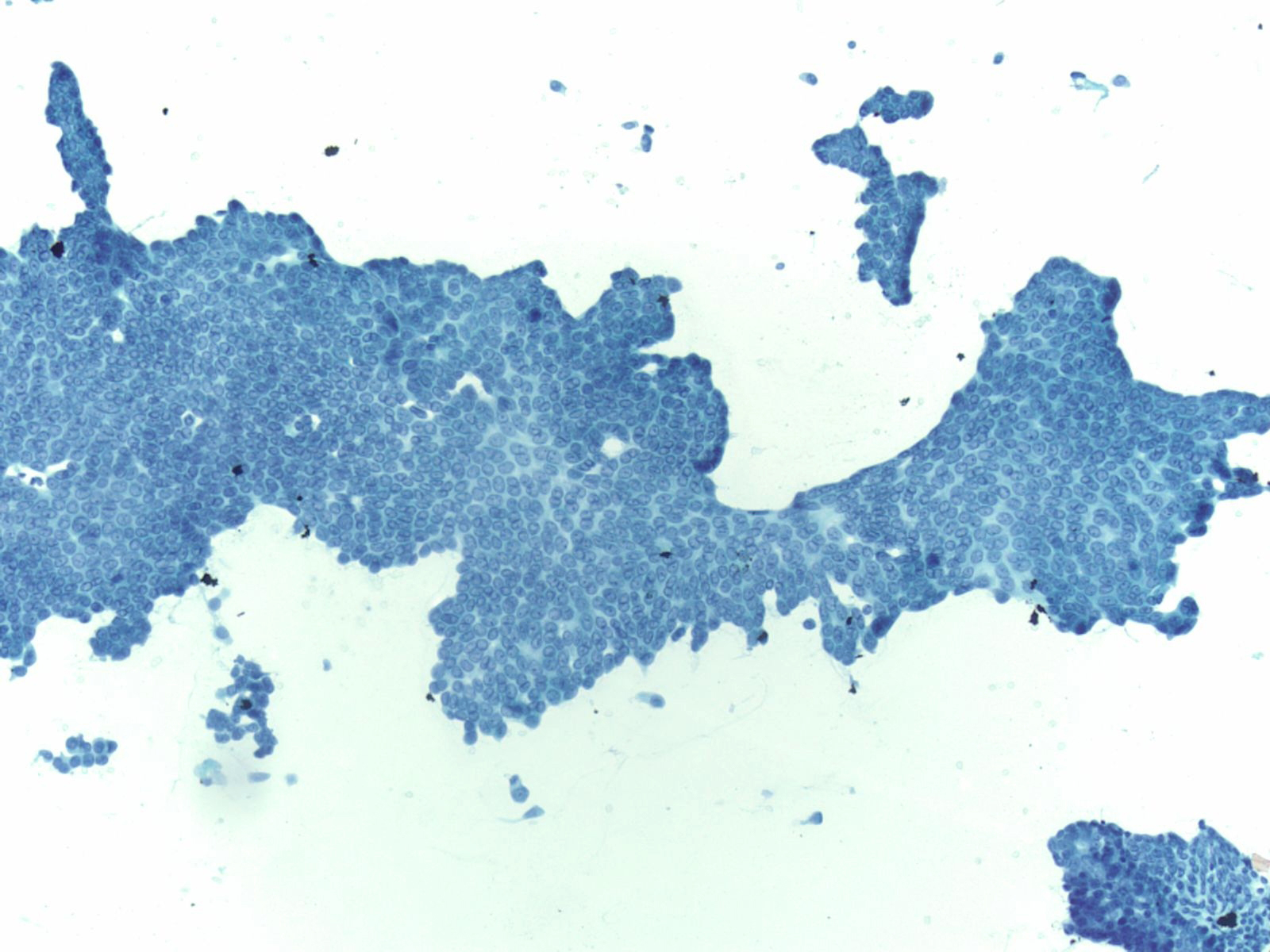 |
||
Well-Differentiated Adenocarcinoma – PPAN3-29
- Loss of honeycomb architecture with nuclear crowding, overlapping, loss of polarity and uneven spacing ("drunken honeycomb")
- Nuclear area variation (>4:1) within a single group of cells
- Chromatin clearing and/or peripheral clumping (parachromatin clearing)
- Abundant cytoplasmic mucin
- Nuclear contour irregularities, often subtle
Neuroendocrine Tumor –MN07-R09706 and N13-6431
- Incidence: ~2-5% of pancreatic neoplasms; ~50% functional and 50% nonfunctional
- Age, Gender: Any age but most between 40-60 years; M=F
- Prognosis and Therapy: Small neoplasms without adverse prognostic features are curable by surgical resection; prognosis is related to tumor size, mitotic rate, necrosis, extrapancreatic invasion, vascular invasion, and nodal or distant metastases
- Radiological Features: Solid, well-circumscribed masses, usually small (<2cm), but may be large (>6cm); can be cystic
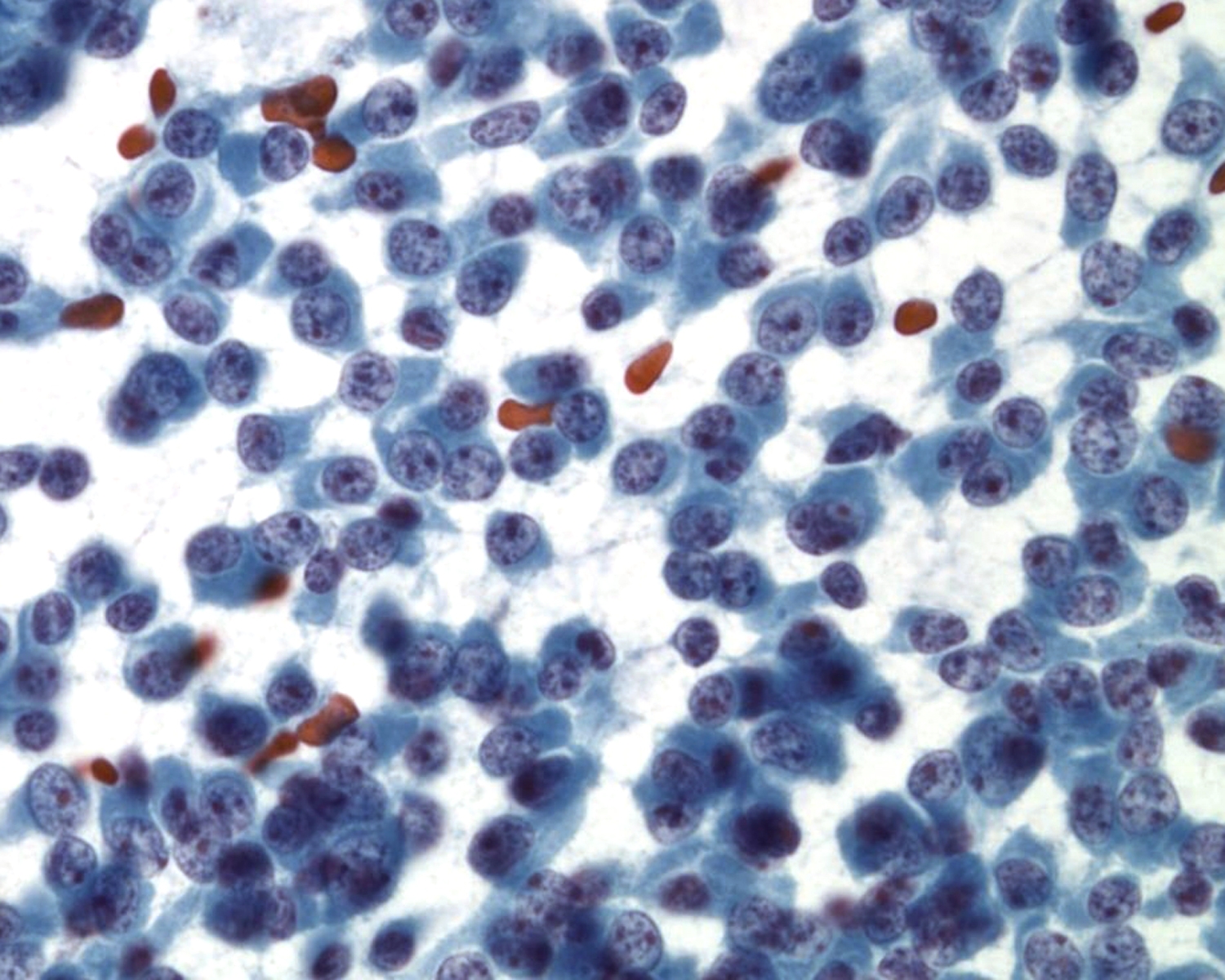 |
||
Key Cytological Features: Pancreatic Neuroendocrine Tumor
- Discohesive, single cell "solid-cellular "smear pattern
- Uniform, monotonous population of cells with plasmacytoid features
- Coarse, speckled, “salt and pepper” chromatin pattern
- Nucleoli may be prominent
- Dense, finely granular cytoplasm
Acinar Cell Carcinoma – PAN2-036
- Incidence: ~1-2% of adult pancreatic neoplasms
- Age,Gender: children<<adults; peak incidence, 7th decade; M:F:: 4:1
- Prognosis and Therapy: directly related to tumor stage and is better than for ductal adenocarcinoma. Resection is treatment of choice.
- Radiological Features: Solid masses with well demarcated borders; rarely cystic
Key Cytological Features: Acinar Cell Carcinoma
- Solid-cellular smear pattern of monomorphic cells
- Cellular clusters of various sizes and single cells (loss of organoid "grape-like Clustering of benign acinar tissue)
- Stripped naked nuclei; +/- loose cytoplasmic granules (best noted on Hand E stain)
- May be disarmingly bland, with a polygonal cell shape and low N:C
- Coarse chromatin usually with prominent nucleoli, but not always granular cytoplasm, variably prominent
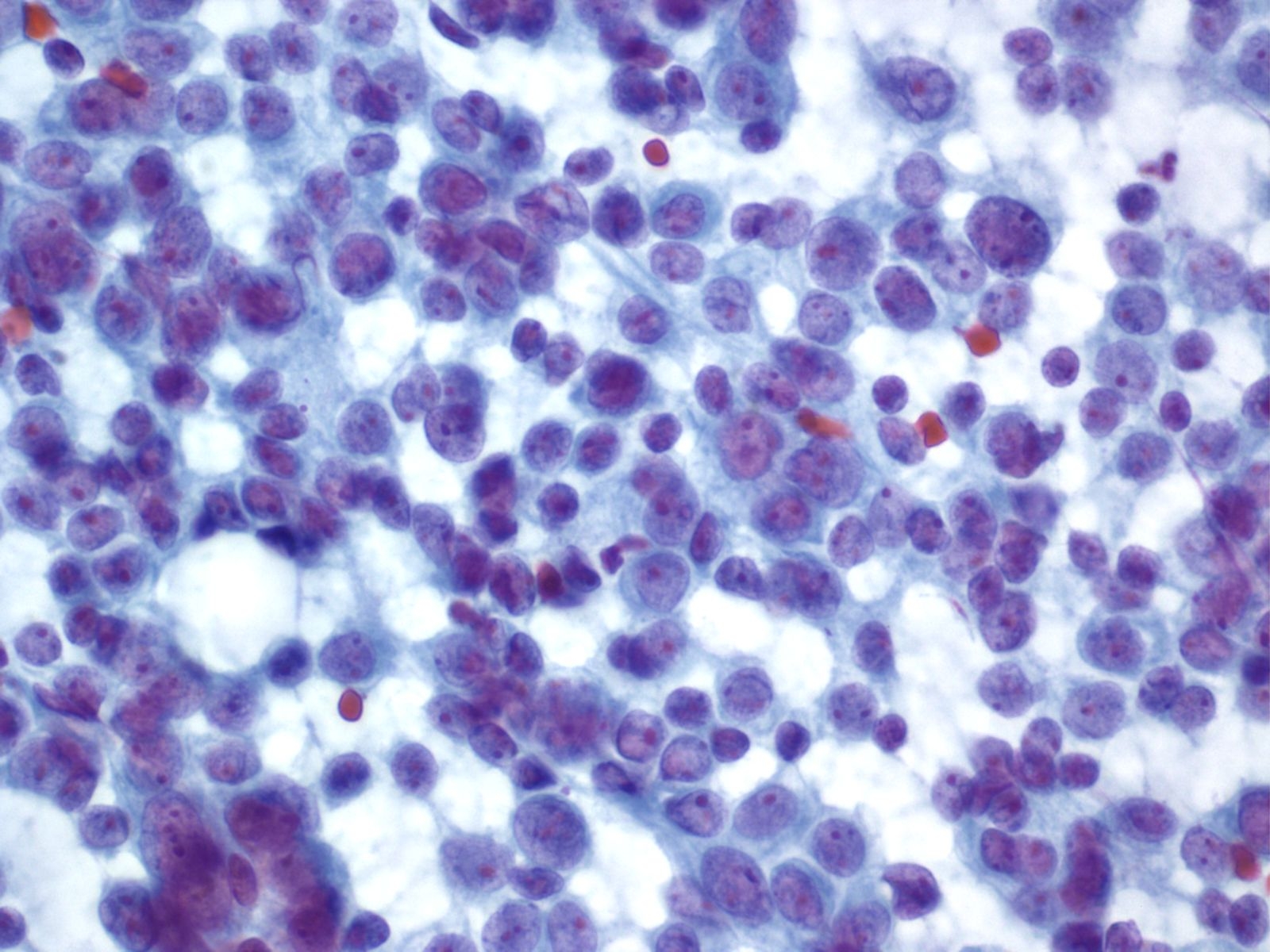 |
||
Solid Pseudopapillary Neoplasm [SPN labeled slides]
- Incidence: ~1-3% of all pancreatic malignancies; 6% of exocrine tumors and ~24% of all surgically resected cystic lesions in the pancreas
- Age, Gender: ~90% women are in their 20’s; mean age is 28 years
- Prognosis and Therapy: Prognosis is excellent with only 15% developing recurrence or metastases
- Radiological Features: Well-circumscribed mass with solid and cystic components, usually in the pancreatic tail
Key Cytological Features: Solid-Pseudopapillary Neoplasm
- Solid cellular smear pattern with or without branching and papillary cell clusters
- Fibrovascular myxoid stromal papillae are characteristic Cells are relatively bland with little anisonucleosis and no mitotic activity
- Nuclei are round to oval with frequent nuclear grooves or focal indentations and finely granular chromatin and inconspicuous nucleoli
- Cytoplasm is typically scant and ill-defined but can be moderate with small perinuclear vacuoles or intracytoplasmic hyaline globules, best detected on air-dried smears
- Smear background may be clean or filled with hemorrhagic cyst debris, foamy histiocytes and multinucleated giant cells
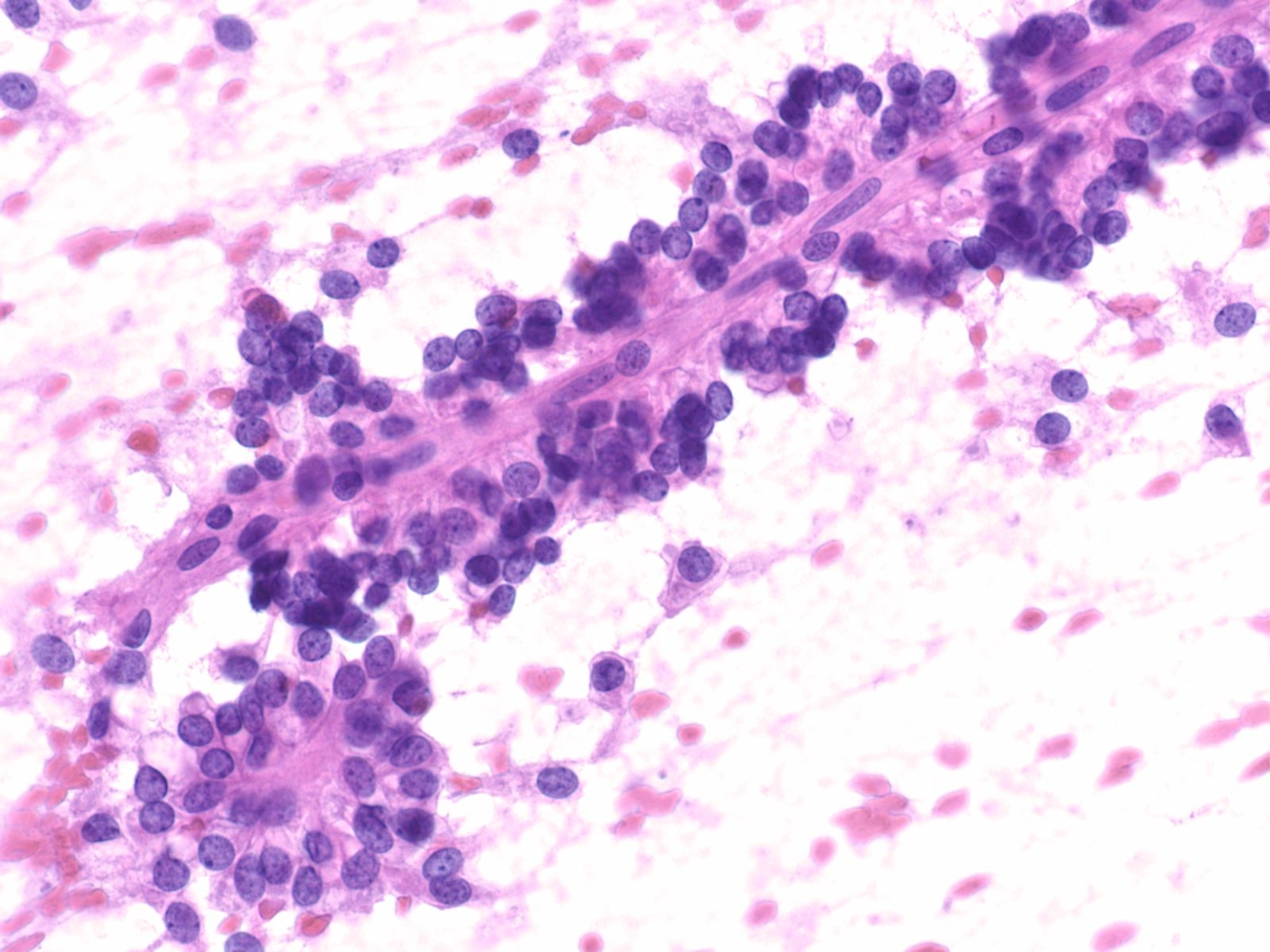 |
||
Cytology service page
Warning: Display title "1-12 Kidney FNA: R Tambouret MD, E Brachtel MD, R Arpin CT" overrides earlier display title "1-11 Pancreas FNA: M Pitman MD, J Misdraji MD, M Nutter CT".
Indications for Kidney Fine Needle Aspiration Cytology/Biopsy
Procedure — Biopsy is usually performed as an outpatient procedure under CT or US guidance with conscious sedation. At MGH a coaxial approach is used which entails per cutaneous insertion of a sheath with stylet (e.g. 10 cm coaxial 17 gauge Temno needle) into the lesion. The stylet is then removed and smaller needles are inserted to obtain fine needle Aspiration cytology and 18 gauge core biopsies. Percutaneous biopsy is safe with a very low risk of clinically significant bleeding or seeding of the needle tract with malignant cells. Rapid on site evaluation (ROSE) by the cytology team is rarely requested at MGH unless a concurrent ablation is planned. Usually though the ablation is done as a second procedure after the FNA. Exceptionally the FNA may be performed by a gastroenterologist with endoscopic ultrasound guidance. The test demonstrates sensitivity and specificity of 80 to 92 percent and 83 to 100 percent, respectively, for the detection of cancer and is especially helpful in differentiating RCC from a metastasis. At MGH, the radiologist will make alcohol fixed smears and will also rinse the needle in an appropriate alcohol solution (e.g. CytoRichRed for SurePath processing). In other practices may also pathologists may request air-dried smears for Giemsa staining in addition to alcohol fixed slides. The core biopsy is fixed immediately in 10% formalin.
At MGH, the radiologist will make alcohol fixed smears and will also rinse the needle in an appropriate alcohol solution (e.g. CytoRichRed for SurePath processing). In other practices may also pathologists may request air-dried smears for Giemsa staining in addition to alcohol fixed slides. The core biopsy is fixed immediately in 10% formalin
The cytology report includes a statement of adequacy (adequate, evaluation limited by ...., unsatisfactory), the interpretation category (No malignant cells, Atypical, Suspicious for malignancy, Positive for malignant cells) and the Diagnosis (there are some drop down menu choices or the pathologist can free text the diagnosis). The core biopsy diagnosis is included under the cytology N number as part B. Currently Two H&E ribbons are produced for the core biopsy.
Basic Cytomorphology
- Proximal Convoluted Tubule (PCT): tubules and sheets, abundant granular cytoplasm, ill-defined fragile cytoplasm without cell borders (unlike oncocytoma)
- Bland nucleus, prominent nucleolus, mimics: oncocytoma, RCC
- Distal Convoluted Tubule/collecting Duct (DCT/CD): tubules, flat sheets (unlike RCC)
- Well-defined cytoplasm
- Smaller cells, no vacuoles (unlike RCC)
- No nucleolus, mimic: RCC
- Capillary loops best seen on the periphery of the cell group
- Very cellular, spindled and round cells in a crowded structure, the glomeruli may retain their round shape or may be drawn out into an irregular or ovoid shape during smearing
- No atypia
- No spherules or papillae (unlike papillary RCC)
- Beware of a smear containing both glomeruli and tubules, the radiologist may have missed the lesion or the abnormality seen on imaging may * be non-neoplastic
- Clear Cell Type (CRCC): clean or necrotic background
- Cohesive monolayered sheets or crowded groups with a mixture of cells with granular cytoplasm and cells with clear or finely vacuolated cytoplasm (clear cells may be in the minority) (unlike oncocytoma)
- Prominent branching capillaries traverse the crowded cell groups
- Usually round nuclei, variable nucleoli
- Impox: Vimentin, cytokeratin, CD10, RCC, PAX8, PAX2 positive
- Hale's colloidal iron negative
- MIMICS: glomeruli, distal convoluted tubule and collecting duct, oncocytoma, chromophobe RCC
- Chromophobe Type: sheets, clusters, single cells with granular cytoplasm
- Bare nuclei (unlike oncocytoma)
- More variation in cell & nuclear size than oncocytoma
- Vesicular round nuclei, binucleation, inclusions, irregular nuclear outline prominent nucleoli in some
- Abundant granular cytoplasm, perinuclear clearing, prominent cell borders ("koilocytic"), fluffy/clear/granular not uniform cytoplasm,
- Impox and special stains: vimentin negative, cytokeratin 7 positive , CD 10 and RCC equivocalHale's colloidal iron positive - uniform, dense, cytoplasmic, (tricky stain, often deceptively pale and uneven in chromophobe) ,: differential diagnosis: oncocytoma, CRCC
- Papillary RCC: small unform cells with scant basophilic cytoplasm (type 1) or more abundant eosinophilic cytoplasm (type 2) in three dimensional spheres or in papillae with cores containing foamy histiocytes; tumor cells and histiocytes may contain hemosiderin
- Impox: AMACR, CD10, RCC positive; CK7 positive more often in type 1 than type 2
- Dyshesive single cells or loose clusters
- round nuclei, smooth borders (but occasional nuclear atypia)
- Binucleation, inconspicuous nucleoli
- Abundant uniformly granular well-defined cytoplasm, no vacuoles
- Vimentin Negative, Cytokeratin 8/18 Positive
- Hale's Colloidal Iron Negative, or Perinuclear/atypical Staining Present, MIMICS: PCT, Chromophobe RCC, Conventional RCC with Granular Cytoplasm
Warning: Display title "1-13 Adrenal: R Tambouret MD, E Brachtel MD, R Arpin CT" overrides earlier display title "1-12 Kidney FNA: R Tambouret MD, E Brachtel MD, R Arpin CT".
- Indications for cytology examination
- Procuring the specimen
- Test platforms/specimen processing and triage
- Report
Basic cytomorphology
- Variable Cellularity
- Cortex:
- Bare nuclei with foamy lipid from stripped cytoplasm background
- Single cells with round nuclei, inapparent nucleoli
- In intact cells, abundant vacuolated cytoplasm with frayed edges
- Medulla
- Rarely sampled, cells have basophilic cytoplasm
- Large eccentric nucleus with fine chromatin
- Conspicuous nucleoli
Adrenal cortical nodules (hyperplastic, adenomas)
- Usually indistinguishable from benign cortical cells
- Loosely cohesive round/plasmacytoid to spindled cells
- Fibrillary cytoplasm, oval hyperchromatic nuclei - may show anisonucleosis/pleomorphism/binucleation
- Melanin pigment in some cells
- Red cytoplasmic granularity on air dried material
- Synaptophysin, chromogranin positive; S-100 positive in spindle cells
- MIMICS: Adrenal cortical carcinoma, other poorly differentiated malignancies
- May not be able to diagnose on cytology alone, but may suspect if mitoses, necrosis or marked pleomorphism is present
Histological assessment required to distinguish larger adenomas from carcinomas
- cell population resembling primary tumor
Cytology service page
Warning: Display title "1-14 Breast FNA: E Brachtel MD, A Ly MD, D Tetreault CT" overrides earlier display title "1-13 Adrenal: R Tambouret MD, E Brachtel MD, R Arpin CT".
Breast lesions can be detected on physical examination by the patient or a physician, or by screening mammography. By palpation, benign masses tend to be soft, rubbery, and mobile with well defined margins. Masses that are hard or fixed with ill-defined or irregular borders are worrisome for malignancy. Mammography is the standard diagnostic imaging modality, but in younger women with dense breast tissue or postmenopausal women on hormone replacement therapy, mammography has limited utility and ultrasound needle used instead. As women age, their breasts are progressively replaced with fat and become less dense, enhancing the diagnostic accuracy of mammography. During pregnancy, ultrasound is used to evaluate breast masses so that radiation exposure can be avoided and also because mammography is less useful in the hormonally altered breast tissue where the amount of adipose tissue is decreased. For men, a combination of mammography and ultrasound is commonly used.
Fine needle aspiration (FNA) is a minimally invasive, cost effective means of evaluating breast masses detected by palpation or by radiographic imaging. The procedure is very well tolerated and can be performed in the clinic setting without anesthesia. Multiple studies have demonstrated the utility of FNA biopsy in evaluating breast lesions. However, the sensitivity (78% to 99%) and specificity (79% to 99%) of breast FNA vary widely among institutions. The rates in some studies are comparable to those for core needle biopsy, whereas other rates are less than optimal and support the use of core needle biopsy instead of FNA. The best results are obtained when the FNA biopsy is performed by skilled aspirators with on site pathologists who are available for adequacy evaluation and experienced pathologists to interpret the specimens. It is important that physicians who perform FNA biopsies receive supervised training in the technique. Formally trained physicians who have performed more than 150 supervised FNA biopsies have significantly lower false-negative and non-diagnostic rates been due informally trained physicians who have performed fewer than 10 supervised FNA biopsies and learned the technique through reading a description, attending a lecture, or by observing the procedure.
Diagnostic accuracy is greatly improved when the FNA results are correlated with clinical and radiologic findings (triple test). The use of the triple test decreases the false negative rate from 10% to less than 1% and the false positive rate from 1% to less than 0.2%. The most common cause of a false negative result is sampling error, which can be due to causes such as small lesion size, fibrosis, and/or inexperience of the aspirator. In contrast, a false positive result is commonly due to interpretive error and can involve hyperplastic processes such as fibroadenoma, proliferative fibrocystic change, papillomas, and lactational change. If there are discordant results between the clinical, radiographic, and/or FNA findings, excisional biopsy is recommended for further evaluation, especially in cases where definitive therapy is planned for a malignant cytologic diagnosis.
FNA causes few complications, most commonly temporary pain or bruising at the biopsy site. Hematomas can occur but are preventable by applying adequate pressure after obtaining the sample. Infection is uncommon. Rare instances of needle track seeding by tumor cells have been reported when samples are taken was larger bore needles (14- to 18-gauge) than are typically used for FNA biopsy (23- to 25-gauge). Pneumothorax is another rare complication and tends to occur more frequently in women with slight builds, smaller breasts, and deeper lesions. It can be avoided by keeping the path of the needle angled to the rib cage and not inserting the needle perpendicular to the chest wall. In some instances, FNA biopsy has been reported to cause infarction of the lesion, most commonly in papillomas and fibroadenomas, which can lead to diagnostic difficulty when evaluating subsequent excision specimens.
Once the mass is fixed with the non-aspirating hand, the skin is cleaned with an alcohol swab at the planned needle entry site. The loosened needle cap is removed and the aspirating hand stabilized by resting the syringe barrel against the thumb or forefinger of the non-aspirating hand. This guards against any physiologic hand tremor and ensures precise needle placement, but is not be needed after insertion of the needle. The needle is inserted into the lesion and the syringe plunger pulled back to generate several cubic centimeters of vacuum. The vacuum is maintained until the needle is removed from the patient. With a straight wrist, the needle is moved back and forth quickly and repeatedly in a sawing motion (“excursions”) for a dwell time no longer than 15-20 seconds (approximately 40-60 excursions) along the original needle trajectory, alternately advancing into the mass and withdrawing to a superficial location without exiting the patient. Slower needle action will yield less material. A shorter dwell time (2 to 5 seconds) is recommended for highly vascular lesions. Each time the needle advances into the lesion, its cutting tip dislodges small tissue fragments; this cutting action is essential for a successful FNA. Negative pressure alone without needle movement will not procure enough tissue for diagnosis in solid lesions.1 The vacuum in the syringe helps conduct the tissue fragments into the needle shaft and hub. A slight acceleration of the needle as it advances into the mass enhances the cutting action of the needle tip. Keeping the needle tip within the mass avoids diluting the specimen with adjacent non-lesional tissue. Material can be seen accumulating in the needle hub, although absence of visible material does not signify an inadequate sample. If blood is rapidly entering the hub, withdraw the needle immediately.
Sampling with thinner needles (25g or 27g) is preferred for vascular lesions as well as for fibrous lesions like a fibroadenoma. When sampling a sclerotic lesion, the needle should be moved more vigorously. To sample more of the mass with one needle pass, withdraw the needle tip to a superficial location while maintaining vacuum, then redirect it to a different area by changing the angle of entry. “Fanning” allows for sampling of a larger area: after each excursion, when the needle tip is in a superficial location, change its angle of entry slightly until the entire region of interest is sampled. Avoid changing direction when the needle is deep in the lesion. This results in tissue tearing and hemorrhage, which compromises the diagnostic yield of subsequent passes.
Remove the needle from the patient after the last excursion is completed or when material or blood is visible in the needle hub, which can occur in less than 15 seconds. Withdraw the needle from the patient in a controlled manner and only after you have released the vacuum in the syringe. Failure to release suction before withdrawing the needle from the patient pulls the aspirated material into the barrel of the syringe, making it difficult to expel for smear preparation. Pressure to the site is applied immediately with gauze to minimize bleeding. The patient or an assistant should perform this step, because the aspirator needs to prepare the sample immediately.
An FNA procedure typically involves inserting the needle into the mass 2 or 3 times (“passes”) to obtain several samples. The center of the mass is often sampled on the first needle pass with the needle approximately perpendicular to the skin. Other areas of the mass are sampled on subsequent passes, especially if the initial material is necrotic, cystic, or otherwise non-diagnostic. Sampling the mass along its long axis tends to yield more cellular specimens compared with moving the needle along the short axis.
As the FNA needle penetrates the target mass, the aspirator can learn to assess the texture of the lesion. Various adjectives used to describe the texture include gritty, rubbery or leathery, and soft. Learning to "feel with the needle" in this way can yield very useful information about the lesion being sampled. Masses are often heterogeneous, and different regions not only feel different but may also yield different cytologic material.
All breast cytology specimens (FNA, duct lavage, or nipple discharge) may be prepared as direct smears (air dried or alcohol fixed) or as liquid based thin layer slides (ThinPrep or SurePath). In general it is desirable to prepare both alcohol fixed and air dried smears; fixed smears are useful in the evaluation of nuclear detail, whereas air dried smears allow for easier identification of cytoplasmic quality and background features such as mucin and stroma. Liquid based techniques are best for specimens such as cyst fluid or duct lavage, which consist of a considerable volume of fluid. The aspirated material can be directly placed in to a vial of liquid based fixative.
If liquid based preparations are used, it should be noted that the processing required to make a thin layer slide can alter the specimen appearance from what is typically seen on a direct smear. It is important to be aware of the differences because the cytologic features used to support a diagnosis of malignancy differ in direct aspirate smears and liquid based preparations.
Cell blocks are useful for cases requiring immunohistochemistry for hormone receptor status or in situ hybridization for HER-2/neu. Because most immunohistochemistry laboratories have validated their stains for formalin fixed paraffin embedded tissue, it is best to place the FNA material directly into formalin. Alternatively, it can be transported in saline or Roswell Park Memorial Institute (RPMI) medium prior to fixing in formalin. It is less than optimal to prepare a cell block from material placed in liquid based fixative such as CytoLyt or CytoRich Red because typically the stains are not validated for these alternative alcohol based fixatives. The easiest method for making cell blocks is to allow bloody specimens to clot in the hub of the needle before flicking the material onto a glass slide and then rinsing the material into formalin. For nonbloody or non-clotted specimens, the material can be rinsed directly into formalin, but multiple visible particles are needed to prepare an adequate cell block. In these cases, addition of HistoGel or use of a collodion bag may be necessary to prevent loss of material during processing. To ensure that adequate material is obtained it is best to evaluate immediately a smear prepared from a drop of material from each aspirate sample.
Standard reporting of breast FNA results uses 5 managerially relevant categories: No malignant cells identified, atypical, suspicious for malignancy, positive for malignant cells, and non-diagnostic/unsatisfactory. These were developed in 1996 as part of the National Institute of Health practice guidelines, referred to as the Uniform Approach to Breast Fine Needle Aspiration Biopsy. The benign category encompasses benign breast tissue, simple cysts, fibrocystic change, fibroadenoma, fat necrosis, lactational change, and inflammatory processes. The atypical/indeterminate category applies to cases where the lesion is more likely to be benign but a well differentiated carcinoma cannot be excluded. The suspicious for malignancy category is used when the lesion is more likely malignant but confirmatory biopsy is recommended before definitive treatment. The malignant category should be reserved for cases that are definitely malignant, the majority of which consist of primary breast adenocarcinomas (ductal and lobular). The atypical/indeterminate and suspicious for malignancy categories apply to cases where the FNA results are inconclusive. The distinction between these 2 categories can be somewhat subjective. A general statement regarding the likelihood of malignancy should be indicated in the report, but both atypical and suspicious diagnoses warrant a surgical biopsy. The unsatisfactory category is for specimens that are determined to be inadequate due to insufficient sampling or preparation artifact.
In general, determination of adequacy relies on assessment by the aspirator as to whether the sample is representative of the palpated or imaged lesion and by the pathologist as to whether the slides are well prepared and interpretable without significant artifacts of slide preparation such as air drying, obscuring blood, or overly thick smears. Therefore it is helpful if the aspirator and the interpreter are the same person and that person is able to review the sample for adequacy at the time the FNA procedure is completed. In this way, additional sampling may occur promptly, and the likelihood of obtaining an adequate specimen increases.
Basic cytomorphology
- Normal ductal cells form a single cell layer attached to a basement membrane. The 2-dimensionally arranged ductal cells are cohesive with each other
- Myoepithelial cells are another cell type that normally lies between the basement membrane and the ductal cells. They typically have slightly darker nuclei, and paler fragile cytoplasm that may rupture when the ductal cells strip from the basement membrane
- Apocrine cells have a prominent nucleoli, round nucleus with an abundant granular cytoplasm; exhibit cohesiveness while maintain their cell boarders.
- Adipocytes are large clear cells with well-defined cytoplasmic boarders, comparable to “soap suds”
- Foam cells or macrophages are inflammatory cells with hypervacuolated cytoplasm and a reniform shaped nuclei
- Ductal cells are arranged in a "staghorn" or animal shapes or blunt branched duct configuration.
- Scattered stripped myoepithelial cells in the background
- Identification of stromal tissue fragments is important for a definitive diagnosis of fibroadenoma
- The fibromyxoid stromal fragment often has a smooth surface and bland morphology of the mesenchymal nuclei
- Few to no epithelial cells- possible fragments of fibroadipose tissue
- No single epithelial cells
- Inflammatory cells, single histiocytes and giant multinucleated histocytes
- Relatively clean background
- Cellular aspirates, benign ductal cells in papillary groups that can mark atypia and necrosis in the presence of infarction
- Myoepithelial cells are present indicating the benign nature
- Apocrine metaplasia may be seen
- If enough material is available, immunostains for myoepithelial markers (e.g. p63) can help to recognize the myoepithelial cell population that underlies the ductal cells; excision may be necessary to definitively determine the nature of a papillary lesion on cytology
- Low to moderately cellular specimen which can contain group of ductal cells
- Ductal cells have small rounded nuclei with little pleomorphism
- Scattered myoepithelial cells
- Not much different from a female breast sample
- Contain a monotonous neoplastic cell population of papillary groups and single cells; cells with randomized polarity forming smooth, rounded spaces
- Necrotic debris
- Areas of possible cribriforming and fibrovascular cores
- Represents a biphasic breast tumor of uncertain malignant potential
- Both stromal components and ductal epithelial cells may be present
- Low grade phyllodes tumors often resemble fibroadenomas on cytology
- High grade malignant phyllodes tumors resemble sarcomas and may show bizarre spindle cells singly and in clusters on cytology
- In those cases, nuclear atypia is prominent, nuclei are plump and a small amount of wispy cytoplasm is present
- Discohesive monotonous ductal cell population with randomized polarity, 3D groups and single cells
- Nuclei containing prominent nucleoli, nuclear boarders are irregular atypical chromatin
- Apoptotic bodies, necrotic debris
- Absence of myoepithelial cells
Immunostaining for HER2/c-erbB-2 is done on cell blocks and can show overexpression of the HER2 protein. Fluorescence in situ hybridization (HER2-FISH) may show that the HER2 gene is amplified. If the test results indicate HER2 overexpression and/or HER2 amplification, then a patient may be eligible for treatment with the cancer drug Herceptin® (Trastuzumab)
- Aspirates may be sparse but can also be cellular
- Tumor cells tend to be small with eccentric nuclei
- Nuclei are mostly uniform in size but may be irregular, with small nucleoli
- Lobular-type tumor cells may show targetoid intracytoplasmic mucin vacuoles
- Thin cords of lobular carcinoma cells can infiltrate the adipose tissue, "single file" pattern
A cytokeratin immunostain can be helpful to disclose the sparse and inconspicuous lobular carcinoma cells in aspirates, or in cavity fluids
- Syncytial groups of anaplastic tumor cells with delicate cytoplasm
- Numerous malignant nuclei, often bare and devoid of cytoplasm
- Lymphocytes and necrosis may be present in the background
- The definitive diagnosis of medullary carcinoma of the breast depends on architectural features, which can only be made on surgical pathology specimens
“Triple Test” for Breast Lesions
- Has a high negative predictive value if all three modalities of a breast lesion are negative: clinical impression, imaging findings and tissue diagnosis, often by cytology
- Cytology findings have to be correlated with both clinical and imaging findings. If a breast sample appears benign on cytology but is clinically or radiologically suspicious – this is a discorrelation
- Do not mix up with “triple negative” breast cancer, which refers to breast cancers that are negative for estrogen and progesterone receptors, and HER2 proteins
Reference- McKee, Grace T. Cytopathology of the Breast, MGH 2002
Fisher, Andrew H. Breast FNA, Cytologystuff through Hologic, 2013
Cytology service page
Warning: Display title "1-15 Lymph Node FNA: I Chebib MD, R Tambouret MD, D Kuebler CT" overrides earlier display title "1-14 Breast FNA: E Brachtel MD, A Ly MD, D Tetreault CT".
- Indications for cytology examination
- Procuring the specimen
- Test platforms/specimen processing and triage
- Report
Basic cytomorphology
- Abundantly cellular smears with polymorphous population of lymphocytes
- Background lympho-glandular bodies
- Small round mature resting lymphocytes from the mantle zone about 12 μm in diameter (compare to RBC 7 μm diameter) with blocky chromatin and scant rim of cytoplasm;
- Polymorphous germinal center cells
- Small and large cleaved follicular center cells with twisted nuclei with inconspicuous nucleoli in the small cells and larger cleaved cells about 2x size of the small lymphocytes and with one or two small
- Scattered larger centroblasts about 2-3x larger than small lymphocytes with finely dispersed chromatin, multiple nucleoli and basophilic cytoplasm
- Phagocytic tinglible body macrophages and many epithelioid histiocytes
- Lymphoid tangles with high endothelial venules
- Follicular dendritic cells with large round pale nuclei and abundant wispy cytoplasm, often seen as 2 cells side by side like “snowmen”
- Ancillary studies are often performed from the FNA sample and include culture and flow cytometry. A preliminary evaluation of one smear will guide triage of the sample.
- Large multinucleated giant cells
- Aggregates of epithelioid histiocytes with ovoid nuclei or elongate twisted nuclei
- Fine chromatin and small nucleoli c/w granulomata, best seen on smear but also on SP
- Consistent with a granulomatous inflammation
- AFB and GMS negative
- B-cell lymphoma, consistent with small lymphocytic lymphoma/CLL
- Abundant small mature appearing lymphocytes, some with nuclei
- Rare larger lymphocytes consistent with prolymphocytes
- No evidence of germinal centers
- Flow cytometry: DIFFERENTIAL COUNT (light scatter): Lymphocytes :94%
LIGHT SCATTER GATE ANALYZED: Lymphocyte T cell total: 16% B cell total 82% CD19 82% CD20 56% kappa 82% INTERPRETATION: CD19+, CD20+, CD23+, CD43+, CD5+ B cells with monotypic expression of lambda light chain. Note: This immunophenotype is consistent with chronic lymphocytic leukemia.
- Lymph node excision (S00R692): B-CELL CHRONIC LYMPHOCYTIC LEUKEMIA/SMALL LYMPHOCYTIC LYMPHOMA
- Mainly small lymphocytes with round to clefted nuclei, small nucleoli and chromatin more finely dispersed than small mature lymphocytes
- Scattered lymphoid tangles c/w follicle centers.
- Flow cytometry shows the sample is composed of 89% lymphocytes with an abnormal population of CD19+, CD20+, CD43-, CD10+, CD23+, CD5- B-cells with monotypic lambda expression
- Consistent follicle center lymphoma. (Grading of follicular lymphophomas on cytology is controversial as the architectural landmarks are usually required.)
- Core biopsy (MS06-14879): FOLLICULAR LYMPHOMA,GRADE 1/3.
- NOTE: The cores comprise lymph node tissue with preserved sinuses but numerous abnormal small to medium-sized follicular structures. These are composed predominantly of small, slightly irregular lymphocytes. Centroblasts are infrequent (<5/HPF)
- Highly cellular sample with numerous discohesive large single cells
- Note the clefted, irregular nuclei, prominent nucleoli and little cytoplasm
- Large cell lymphomas will be 2-5X the size of normal lymphs
- Tingible body macrophages are present due to high turnover of the tumor cells, don’t confuse with reactive germinal centers
- Cell clustering is absent
- On the air dried sample, note the enlargement and the difference in the chromatin pattern compared to the alcohol fixed Papanicolaou stain smear
- Flow cytometry may be falsely negative because the large lymphoma cells are fragile and may not survive processing.
- Abundant necrosis, debris and numerous predominantly single relatively small malignant cells with little to no cytoplasm, dark finely granular chromatin (unlike the background lymphocytes)
- Tumor cells do not have prominent nucleoli and demonstrate nuclear molding
- Immunohistochemistry performed on the cellblock confirmed diagnosis: TTF1 +, synaptophysin and chromogranin rare + cells
Cytology service page
Warning: Display title "1-16 Bone and Soft Tissue Cases: I Chebib MD, D Kuebler CT" overrides earlier display title "1-15 Lymph Node FNA: I Chebib MD, R Tambouret MD, D Kuebler CT".
General
The evaluation of a soft tissue mass by fine needle aspiration remains controversial. There are a number of reasons for this that include:
1) Soft tissue tumors are relatively rare and sarcomas are generally only treated at tertiary care centers;
2) Cytopathologists without a background in soft tissue and bone pathology may be uncomfortable with interpretation of these rare specimens;
3) There is general perception that soft tissue tumors, especially benign and low-grade lesions do not yield sufficient tissue for interpretation and ancillary testing;
4) A growing number of soft tissue tumors have very similar cytomorphologic features that cannot be adequately differentiated on cytology or even histology alone;
5) There is also a growing number of ancillary tests (immunohistochemistry, fluorescence in situ hybridization, cytogenetics, electron microscopy, polymerase chain reaction) required for the appropriate categorization of soft tissue tumors. Cytology may not yield sufficient tissue for the growing array of ancillary tests needed.
6) Not uncommonly, soft tissue tumors may require the opinion of pathologists with expertise in bone and soft tissue pathology who themselves may not be comfortable with interpretation of cytology specimens.
7) Many deep soft tissue and bone tumors are biopsied by interventional musculoskeletal radiologists who may not be trained or experienced in procuring appropriate cytology specimens;
Despite these concerns, there are many situations where fine needle aspiration (FNA) biopsy of soft tissue or bone tumors may be appropriate. The most common situations for FNA biopsy of soft tissue and bone tumors include:
1) Clinical/radiologic impression of metastatic carcinoma, melanoma or high-grade lymphoma to bone or soft tissue. Cytology for epithelial, melanocytic or high-grade lymphoproliferative malignancy has been well established and interpretation by a cytopathologist is relatively straightforward if sufficient material can be procured. Many of these situations require biopsy by interventional radiology and the use of rapid on-site evaluation (ROSE) assists in ensuring these specimens have sufficient diagnostic tissue for interpretation.
2) FNA biopsy in patients with a previously established sarcoma diagnosis. In these patients, a known diagnosis aids in the interpretation of sarcomas of bone, soft tissue, lung etc. where metastasis from a known sarcoma primary may be aspirated to confirm metastasis. Comparison to the previous surgical pathology specimen of the primary sarcoma aids in interpretation of these specimens and typically does not require the addition of a large number of ancillary tests.
3) FNA biopsy of primary soft tissue and bone tumors. This situation, as addressed above, is the most controversial. However, in some situations, this may be the most viable option. Superficial tumors may be sampled in an FNA clinic relatively quickly, not necessarily to establish a specific diagnosis but to triage the patient to a general category: infectious versus neoplastic, benign versus malignant, or even morphologic subcategorization: spindle cell versus small round blue cell versus epithelioid versus pleomorphic etc. Finally, soft tissue and bone tumors can be biopsied via FNA for primary diagnosis, if the appropriate tissue is available for ancillary testing.
References
1. Kilpatrick SE1, Cappellari JO, Bos GD, Gold SH, Ward WG. Is fine-needle aspiration biopsy a practical alternative to open biopsy for the primary diagnosis of sarcoma? Experience with 140 patients. Am J Clin Pathol. 2001 Jan;115(1):59-68.
2. Liu K1, Layfield LJ, Coogan AC, Ballo MS, Bentz JS, Dodge RK. Diagnostic accuracy in fine-needle aspiration of soft tissue and bone lesions. Influence of clinical history and experience. Am J Clin Pathol. 1999 May;111(5):632-40.
3. Khalbuss WE1, Teot LA, Monaco SE. Diagnostic accuracy and limitations of fine-needle aspiration cytology of bone and soft tissue lesions: a review of 1114 cases with cytological-histological correlation. Cancer Cytopathol. 2010 Feb 25;118(1):24-32.
4. Singh HK1, Kilpatrick SE, Silverman JF. Fine needle aspiration biopsy of soft tissue sarcomas: utility and diagnostic challenges. Adv Anat Pathol. 2004 Jan;11(1):24-37.
5. Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F (eds.): WHO Classification of Tumours of Soft Tissue and Bone, 4th Edition. IARC: Lyon 2013.
Soft tissue tumors may present as superficial (skin, subcutaneous) or deep (fascial, intramuscular, visceral). Superficial tumors may be biopsied by palpation readily by cytopathologists trained in FNA technique. In fact, aspiration of superficial tumors by a cytopatholigsts trained in FNA allows for immediate assessment of cellularity and typically procurement of sufficient tissue for ancillary testing. However, deep tumors of soft tissue or bone generally require image-guided FNA by interventional radiology. Rapid on-site assessment (ROSE) is very useful in these circumstances, as these tumors are often difficult to obtain sufficient material for interpretation. Bone tumors often require violation and passage of a biopsy needle through cortical bone to achieve sufficient exposure of a medullary tumor, which often complicates the biopsy procedure.
References
1. Kilpatrick SE1, Cappellari JO, Bos GD, Gold SH, Ward WG. Is fine-needle aspiration biopsy a practical alternative to open biopsy for the primary diagnosis of sarcoma? Experience with 140 patients. Am J Clin Pathol. 2001 Jan;115(1):59-68.
2. Liu K1, Layfield LJ, Coogan AC, Ballo MS, Bentz JS, Dodge RK. Diagnostic accuracy in fine-needle aspiration of soft tissue and bone lesions. Influence of clinical history and experience. Am J Clin Pathol. 1999 May;111(5):632-40.
3. Khalbuss WE1, Teot LA, Monaco SE. Diagnostic accuracy and limitations of fine-needle aspiration cytology of bone and soft tissue lesions: a review of 1114 cases with cytological-histological correlation. Cancer Cytopathol. 2010 Feb 25;118(1):24-32.
4. Singh HK1, Kilpatrick SE, Silverman JF. Fine needle aspiration biopsy of soft tissue sarcomas: utility and diagnostic challenges. Adv Anat Pathol. 2004 Jan;11(1):24-37.
5. Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F (eds.): WHO Classification of Tumours of Soft Tissue and Bone, 4th Edition. IARC: Lyon 2013.
FNA of primary soft tissue of bone not only require well prepared and stained alcohol-fixed or air-dried smears but also sufficient material must be procured for cell block preparation, as most primary soft tissue and bone tumors require the judicial use of immunohistochemistry and molecular studies for a definitive diagnosis, with few exceptions. However, communication with the clinician may be helpful as exact subtyping of the tumor may not be required for treatment - FNA may be used as a preliminary assessment for patient triage: ie. infectious/inflammatory versus neoplastic; carcinoma or lymphoma versus sarcoma; benign versus malignant soft tissue tumor, etc. In these situations, a cytomorphologic assessment only may be sufficient to appropriately triage, and in some cases, treat the patient. However, whenever possible, cell block material should be created for potential future requirements and analysis.
References
1. Kilpatrick SE1, Cappellari JO, Bos GD, Gold SH, Ward WG. Is fine-needle aspiration biopsy a practical alternative to open biopsy for the primary diagnosis of sarcoma? Experience with 140 patients. Am J Clin Pathol. 2001 Jan;115(1):59-68.
2. Liu K1, Layfield LJ, Coogan AC, Ballo MS, Bentz JS, Dodge RK. Diagnostic accuracy in fine-needle aspiration of soft tissue and bone lesions. Influence of clinical history and experience. Am J Clin Pathol. 1999 May;111(5):632-40.
3. Khalbuss WE1, Teot LA, Monaco SE. Diagnostic accuracy and limitations of fine-needle aspiration cytology of bone and soft tissue lesions: a review of 1114 cases with cytological-histological correlation. Cancer Cytopathol. 2010 Feb 25;118(1):24-32.
4. Singh HK1, Kilpatrick SE, Silverman JF. Fine needle aspiration biopsy of soft tissue sarcomas: utility and diagnostic challenges. Adv Anat Pathol. 2004 Jan;11(1):24-37.
5. Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F (eds.): WHO Classification of Tumours of Soft Tissue and Bone, 4th Edition. IARC: Lyon 2013.
The reporting of primary soft tissue and bone FNA has not been formalized, like in some other organ systems. In general, a 5-tiered interpretation system is used: non-diagnostic, benign, atypical, suspicious and malignant. However, there are many situations where a clear interpretation may not be possible: definitive neoplastic cells but unclear whether benign or malignant; in these situations "neoplastic cells present" may be used. If the specific subtype of soft tissue tumor is or may be required, production of a cell block is a necessity. Definitive subtyping of the soft tissue or bone tumor typically follows the WHO Classification of Tumours of Soft Tissue and Bone, Fourth Edition (2013).
References
1. Kilpatrick SE1, Cappellari JO, Bos GD, Gold SH, Ward WG. Is fine-needle aspiration biopsy a practical alternative to open biopsy for the primary diagnosis of sarcoma? Experience with 140 patients. Am J Clin Pathol. 2001 Jan;115(1):59-68.
2. Liu K1, Layfield LJ, Coogan AC, Ballo MS, Bentz JS, Dodge RK. Diagnostic accuracy in fine-needle aspiration of soft tissue and bone lesions. Influence of clinical history and experience. Am J Clin Pathol. 1999 May;111(5):632-40.
3. Khalbuss WE1, Teot LA, Monaco SE. Diagnostic accuracy and limitations of fine-needle aspiration cytology of bone and soft tissue lesions: a review of 1114 cases with cytological-histological correlation. Cancer Cytopathol. 2010 Feb 25;118(1):24-32.
4. Singh HK1, Kilpatrick SE, Silverman JF. Fine needle aspiration biopsy of soft tissue sarcomas: utility and diagnostic challenges. Adv Anat Pathol. 2004 Jan;11(1):24-37.
5. Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F (eds.): WHO Classification of Tumours of Soft Tissue and Bone, 4th Edition. IARC: Lyon 2013.
Basic cytomorphology
- Right foot FNA
- Background of amorphous material with scattered histiocytes and multinucleated giant cells
- Polarizable needle shaped crystals seen within the amorphous material
- The findings are consistent with tophus (chronic gout) and the crystals are composed of uric acid
- Compare the appearance of the air dried smear (most cells appear larger in size) to the ethanol fixed Papanicolaou stained smear.
- Bone mass at C-3
- Smear is moderately cellular
- Sheets of large cells with vacuolated cytoplasm
- Round to ovoid nuclei
- Inconspicuous nucleoli
- Physaliferous cells along with the second component of neoplastic cells are smaller with an epithelioid appearance and moderate amounts of an eosinophilic and granular cytoplasm
- The nuclei found in both cell types are variable in size and shape, nucleoli can be prominent
- Mitotic figures are rare
- Marked nuclear hyperchromasia is not present
- A chordoma should always be considered in any soft tissue/ bone/ mass that one encounters
- Pelvic chordomas can present as large lytic lesions in the sacrum and others presenting in the shpeno-occipital area present at a smaller size- these are slow growing malignant tumors
- Chordomas are cytokeratin positive, are reactive with antibodies against epithelial membrane antigen and also S-100 protein
- Shoulder mass FNA
- Smears contain numerous elongated spindle shaped or ovoid nuclei with pale chromatin
- Pale, delicate cytoplasm, mitoses are absent
- Branching and interlacing so called chicken wire network of small vessels present
- Myxoid material staining on the Giemsa stained smear
- Leg mass, smear of tumor
- Classic features of Ewing’s include cellular features with single cells and loose clusters, two cell types, large pale cells with vacuolated cytoplasm and small dark cells with scant cytoplasm
- Numerous naked nuclei due to fragile cytoplasm, and occasional rosette-like structures
- The DDX includes other small cell neoplasms such as lymphoma, neuroblastoma, and small cell carcinoma
- Clinical presentation and patient’s gender and age can assist in making the accurate diagnosis
- This patient’s age is unusual for the typical round cell tumors
- Iliac crest FNA
- Groups of spindly cells with scant cytoplasm and uniform oval nuclei with smooth chromatin
- Multinucleated giant cells with uniform small nuclei (a dozen or more but are fairly uniform in size)
- Mitotic figures may be present
- The nuclei have condensed chromatin with small but distinct nucleoli
- Loosely cohesive small-medium epithelial cells with abundant cytoplasm eccentrically placed oval (sometimes indented) nuclei, and finely granular chromatin
- Several signet ring cells with large cytoplasmic vacuole displacing the nucleus to the side
Cytology service page
Warning: Display title "1-17 Palpable Fine Needle Aspiration Biopsy: Procedure, Techniques, and Specimen Handling<b></b>" overrides earlier display title "1-16 Bone and Soft Tissue Cases: I Chebib MD, D Kuebler CT".
General
Amy Ly, M.D.
Fine needle aspiration (FNA) is widely used for evaluating palpable superficial masses and cysts as well as deep-seated, nonpalpable radiologic abnormalities under image-guidance. The capabilities and limitations of FNA, specific to the evaluation of a specific organ or anatomic site, are discussed in other sections.
In 1930, Martin and Ellis published the first significant North American description of FNA methodology for palpable lesions.1 In spite of the long history of FNA and its application to the care of patients, there is no best practice for performing an FNA, and rigorous comparisons of biopsy techniques are lacking. Although the most common techniques for performing an FNA of a palpable mass are applicable to all superficial sites, there are nuances in method -- some idiosyncratic -- that depend on geographic or institutional custom and/or previous training and experience. Even for individual pathologists, details learned in training are often modified in practice by factors such as height, handedness, hand size, and finger strength.
Hands-on practical training in FNA technique is critical to developing the hand-eye coordination required. When compared with physicians who had no formal training in FNA technique, those who received such training obtained diagnostic samples more frequently.2 The best way to become proficient is to perform procedures under the direct supervision of someone who is proficient and provides feedback. Good training is important, but continued performance of procedures is necessary to maintain competence.
Most complications associated with FNA of superficial sites are similar to those of a blood draw: minor pain, bleeding, and bruising.10 A hematoma develops occasionally. Bleeding is stopped by applying firm pressure to the biopsy site for several minutes. This is sufficient even in patients with a coagulopathy.20 Some patients experience a vasovagal response; positioning the patient supine while performing the biopsy prevents falls in the rare case that a patient faints. If the needle encounters a nerve, the patient may experience radiating/referred pain.21 In such situations, the needle should be removed and the patient observed. The condition is transient and its resolution should be documented in the patient’s record.
More serious complications are rare. A pneumothorax can occur when aspirating a lesion near the chest wall, including the breast, supraclavicular area, and axilla; it occurs in less than 1:200 FNAs. 22-27 The risk is greater in thin patients and can be reduced by choosing a needle trajectory that is tangential to the rib cage. The clinical manifestations are immediate chest and shoulder pain. Mesothelial cells might be seen on the slides.
There have been case reports of death resulting from FNA sampling of a carotid body tumor and carotid artery dissection after “blind” FNA of a neck node.28, 29 Ultrasound guidance can be helpful when sampling near a major vessel.
Seeding of malignant tumor cells along the needle tract has been reported. When large core biopsy needles (e.g., 17g) are used, the rate of needle tract seeding can be as high as 12.5%.30 The risk with FNA, however, is exceedingly low, ranging from 0-0.009%.31-33 The risk increases with the depth of the lesion, needle size, and number of passes.33, 34 Although the limited data available suggest that needle tract seeding by malignant tumor cells does not have clinical implications, disease progression and decreased survival have been ascribed to FNA and core biopsies.35-39 Seeding of the tract by benign tumor cells can also occur.40, 41
FNA procedures pose a risk to the aspirator as well. Whenever the needle tip is exposed, the aspirator is at risk for a needle stick injury. Although the frequency is not well-documented (and likely underreported), the most frequent site of needle stick injury is the index finger of the non-dominant hand (59.6%), which typically occurs during sampling of the lesion.42 Vigilance and attention to the location of the needle tip is important to avoid a needle stick injury. Universal precautions must always be exercised, including wearing a properly fitted N95 mask when performing an FNA on a patient with known or suspected tuberculous infection.
REFERENCES
1. Martin HE, Ellis EB. Biopsy by Needle Puncture and Aspiration. Ann Surg 1930;92(2): 169-81.
2. Ljung BM, Drejet A, Chiampi N, et al. Diagnostic accuracy of fine-needle aspiration biopsy is determined by physician training in sampling technique. Cancer 2001;93(4): 263-8.
3. Guidelines of the Papanicolaou Society of Cytopathology for fine-needle aspiration procedure and reporting. The Papanicolaou Society of Cytopathology Task Force on Standards of Practice. Diagn Cytopathol 1997;17(4): 239-47.
4. Duston MA, Skinner M, Shirahama T, Cohen AS. Diagnosis of amyloidosis by abdominal fat aspiration. Analysis of four years' experience. Am J Med 1987;82(3): 412-4.
5. Libbey CA, Skinner M, Cohen AS. Use of abdominal fat tissue aspirate in the diagnosis of systemic amyloidosis. Arch Intern Med 1983;143(8): 1549-52.
6. Guy CD, Jones CK. Abdominal fat pad aspiration biopsy for tissue confirmation of systemic amyloidosis: specificity, positive predictive value, and diagnostic pitfalls. Diagn Cytopathol 2001;24(3): 181-5.
7. Ansari-Lari MA, Ali SZ. Fine-needle aspiration of abdominal fat pad for amyloid detection: a clinically useful test? Diagn Cytopathol 2004;30(3): 178-81.
8. Westermark P, Stenkvist B. A new method for the diagnosis of systemic amyloidosis. Arch Intern Med 1973;132(4): 522-3.
9. Orlinsky M, Hudson C, Chan L, Deslauriers R. Pain comparison of unbuffered versus buffered lidocaine in local wound infiltration. J Emerg Med 1992;10(4): 411-5.
10. Pitman MB, Abele J, Ali SZ, et al. Techniques for thyroid FNA: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol 2008;36(6): 407-24.
11. Zavala DC, Schoell JE. Ultrathin needle aspiration of the lung in infectious and malignant disease. Am Rev Respir Dis 1981;123(1): 125-31.
12. Kreula J, Bondestam S, Virkkunen P. Sample size in fine needle aspiration biopsy. Br J Surg 1989;76(12): 1270-2.
13. Hoda RS. Non-gynecologic cytology on liquid-based preparations: A morphologic review of facts and artifacts. Diagn Cytopathol 2007;35(10): 621-34.
14. Afify AM, Liu J, Al-Khafaji BM. Cytologic artifacts and pitfalls of thyroid fine-needle aspiration using ThinPrep: a comparative retrospective review. Cancer 2001;93(3): 179-86.
15. Parfitt JR, McLachlin CM, Weir MM. Comparison of ThinPrep and conventional smears in salivary gland fine-needle aspiration biopsies. Cancer 2007;111(2): 123-9.
16. Dey P, Luthra UK, George J, Zuhairy F, George SS, Haji BI. Comparison of ThinPrep and conventional preparations on fine needle aspiration cytology material. Acta Cytol 2000;44(1): 46-50.
17. Tulecke MA, Wang HH. ThinPrep for cytologic evaluation of follicular thyroid lesions: correlation with histologic findings. Diagn Cytopathol 2004;30(1): 7-13.
18. Kulkarni MB, Prabhudesai NM, Desai SB, Borges AM. Scrape cell-block technique for fine needle aspiration cytology smears. Cytopathology 2000;11(3): 179-84.
19. Zajdela A, Zillhardt P, Voillemot N. Cytological diagnosis by fine needle sampling without aspiration. Cancer 1987;59(6): 1201-5.
20. Jadusingh IH. Fine needle aspiration biopsy of superficial sites in patients with hemostatic defects. Acta Cytol 1996;40(3): 472-4.
21. Alkan S, Kosar AT, Erdurak SC, Dadas B. Transient vocal cord paralysis following ultrasound-guided fine-needle aspiration biopsy for a thyroid nodule. J Otolaryngol Head Neck Surg 2009;38(1): E14-5.
22. Mayall F, Chang B. Pneumothorax following fine needle aspiration (FNA) of small axillary lymph node. Cytopathology 1998;9(4): 283-5.
23. Priola AM, Priola SM, Cataldi A, et al. Diagnostic accuracy and complication rate of CT-guided fine needle aspiration biopsy of lung lesions: a study based on the experience of the cytopathologist. Acta Radiol 2010;51(5): 527-33.
24. Catania S, Boccato P, Bono A, et al. Pneumothorax: a rare complication of fine needle aspiration of the breast. Acta Cytol 1989;33(1): 140.
25. Gateley CA, Maddox PR, Mansel RE. Pneumothorax: a complication of fine needle aspiration of the breast. Br Med J 1991;303(6803): 627-8.
26. Goodson WH, 3rd, Mailman R, Miller TR. Three year follow-up of benign fine-needle aspiration biopsies of the breast. Am J Surg 1987;154(1): 58-61.
27. Kaufman Z, Shpitz B, Shapiro M, Dinbar A. Pneumothorax. A complication of fine needle aspiration of breast tumors. Acta Cytol 1994;38(5): 737-8.
28. Ustymowicz A, Guzinska-Ustymowicz K, Kordecki K, Lewszuk A, Krejza J. Carotid artery dissection - an important complication after fine-needle aspiration biopsy. Med Sci Monit 2004;10 Suppl 3: 120-2.
29. Engzell U, Franzen S, Zajicek J. Aspiration biopsy of tumors of the neck. II. Cytologic findings in 13 cases of carotid body tumor. Acta Cytol 1971;15(1): 25-30.
30. Llovet JM, Vilana R, Bru C, et al. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology 2001;33(5): 1124-9.
31. Maturen KE, Nghiem HV, Marrero JA, et al. Lack of tumor seeding of hepatocellular carcinoma after percutaneous needle biopsy using coaxial cutting needle technique. AJR Am J Roentgenol 2006;187(5): 1184-7.
32. Smith EH. Complications of percutaneous abdominal fine-needle biopsy. Review. Radiology 1991;178(1): 253-8.
33. Wu M, Burstein DE. Fine needle aspiration. Cancer Invest 2004;22(4): 620-8.
34. Roussel F, Nouvet G. Evaluation of large-needle biopsy for the diagnosis of cancer. Acta Cytol 1995;39(3): 449-52.
35. Liebens F, Carly B, Cusumano P, et al. Breast cancer seeding associated with core needle biopsies: a systematic review. Maturitas 2009;62(2): 113-23.
36. Rodgers MS, Collinson R, Desai S, Stubbs RS, McCall JL. Risk of dissemination with biopsy of colorectal liver metastases. Dis Colon Rectum 2003;46(4): 454-8; discussion 58-9.
37. Hix WR. Chest wall recurrence of lung cancer after transthoracic fine needle aspiration biopsy. Ann Thorac Surg 1990;50(6): 1020-1.
38. Metcalfe MS, Bridgewater FH, Mullin EJ, Maddern GJ. Useless and dangerous--fine needle aspiration of hepatic colorectal metastases. Br Med J 2004;328(7438): 507-8.
39. Navarro F, Taourel P, Michel J, et al. Diaphragmatic and subcutaneous seeding of hepatocellular carcinoma following fine-needle aspiration biopsy. Liver 1998;18(4): 251-4.
40. Lee KC, Chan JK, Ho LC. Histologic changes in the breast after fine-needle aspiration. Am J Surg Pathol 1994;18(10): 1039-47.
41. Douville NJ, Bradford CR. Comparison of ultrasound-guided core biopsy versus fine-needle aspiration biopsy in the evaluation of salivary gland lesions. Head Neck 2012; 00: 000–000.
42. Kumar N, Sharma P, Jain S. Needle stick injuries during fine needle aspiration procedure: Frequency, causes and knowledge, attitude and practices of cytopathologists. J Cytol 2011;28(2): 49-53.
Additional Resource
Video demonstration of FNA Technique by Dr. Britt-Marie Ljung, Papanicolaou Society website (http://www.papsociety.org/fna.html).
All the equipment needed to perform an FNA (Table 8.1) is small and lightweight enough to be hand-carried in one container. Such portability allows FNAs to be performed on demand and in virtually any setting. The equipment occupies only a small area of counter space when arranged for specimen preparation.
TABLE 8.1 FINE NEEDLE ASPIRATION EQUIPMENT LIST
1. Syringe holder (10 mL Cameco syringe pistol or equivalent)
2. Disposable sterile 10 mL plastic syringes
3. Disposable sterile needles with transparent hubs (23g and 25g, up to 1.5 inches long)
4. Alcohol swabs
5. Sterile gauze pads
6. Glass slides with frosted end for labeling
7. Fluid transport medium (e.g., RPMI, saline)
8. Local anesthesia and needle/syringe to administer (e.g., 2% lidocaine, 27g needle/1 cc syringe)
9. Alcohol fixative (commercial spray fixative or Coplin jar filled with 95% ethanol)
10. Gloves
11. Pen/pencil for labeling slides (e.g., Leica pen, Sakura Tissu-Tek pencil)
12. Plastic slide holders or slide trays (for transporting slides)
Steps for a successful fine needle aspiration (FNA):
Determine if the FNA is warranted Consent the patient for the procedure Position the patient and immobilize the lesion Sample the targeted lesion adequately Prepare the sample for evaluation, including appropriate allocation of material for ancillary studies as necessary Provide post-procedure instructions to the patient Determining if an FNA is Warranted
A patient presenting for an FNA has almost certainly been referred by another physician. The patient’s clinical history should be reviewed when available, preferably before seeing the patient. A focused physical examination should be performed, confirming that the lesion is indeed palpable (and an FNA appropriate) and ensuring that the correct site is aspirated. It is helpful to ask the patient to point to the mass. If the lesion is not palpable or cannot be safely sampled, it should not be aspirated. By reviewing the results of imaging studies and performing a focused physical examination, the aspirator should assess the size, shape, and relationship of the lesion to nearby structures like large blood vessels. Imaging studies document the internal qualities of the lesion (e.g., vascularity and the relative proportion of solid vs. cystic areas) and its distance from the skin surface. This information guides the approach to the lesion (e.g., the length of needle to use). During the brief examination, the aspirator should inquire about a significant bleeding disorder.
Consenting the Patient
The consent process involves a detailed description of the procedure, including its purpose and potential complications, allowing for questions from the patient. The consent form needs to be signed by a witness. (The physician performing the FNA can act as the witness.)
Sample Explanation of the Procedure
“Hello Ms. Doe, I’m Dr. Ly from the Department of Pathology. I understand that Dr. Jones has sent you here for a fine needle aspiration of a mass in your neck. The goal is to determine the diagnosis for the mass. Let me explain what the procedure involves. Feel free to ask questions at any time.
I will use a very thin needle to take a sample of the mass. The needle is the same size or smaller than the ones used to draw blood. I will insert the needle into the mass and move the needle back and forth for about 15-20 seconds. I usually do this 2 or 3 times, which means 2 or 3 separate needle sticks. Sometimes I will need to do it a 4th time if I think I will need additional material to make a diagnosis. I usually take a small break after the 1st or 2nd needle stick and do a quick check with a nearby microscope to see how much material there is. I will not make a diagnosis at this time – I am only checking to ensure that I am in the mass and getting enough material to make a diagnosis.
Most patients feel a pinprick when the needle goes through the skin like during a blood draw, and while the needle is moving back and forth, most patients feel a dull pressure, pulling, or soreness. I can inject lidocaine into the skin over the lump if you like. It will be another small needle and you may feel a burning sensation for a few seconds. If you cannot tolerate the procedure because of pain, I will stop and take the needle out.
This procedure has a few minor risks that you should know about. Bleeding and infection are the most common, but I clean the skin with alcohol before each needle stick to minimize the chances of infection. You will probably experience a small amount of bleeding and possibly bruising. After each needle stick, my assistant will apply firm pressure with a gauze pad to minimize this.
Do you understand the purpose of this procedure and the risks involved, and do you agree to the procedure? If so, please verify for me your full name and date of birth, and I’ll ask you to sign this consent form.”
Readying the Equipment
The biopsy apparatus is assembled by loading a syringe onto the syringe holder and attaching a needle. 22g or smaller needles are considered “fine.”3 Commonly, 23g and 25g needles measuring 1.0 to 1.5 inches long are used for palpable lesions. Larger gauge needles (19g to 22g) are used for aspirating abdominal fat to test for amyloid deposition.4-8 The shortest needle that reaches the furthest area of the lesion from the skin should be chosen. Shorter needles (<1.0 inch long) are sufficient for small nodules close to the skin surface. Needles vary in design; those with beveled tips are preferred, but FNA does not require a specific needle type to be successful. Once set up, the needle cap is loosened, and the equipment placed conveniently. If local anesthesia is to be given, it should be prepared at this time.
Because the sample must be prepared immediately before it dries/clots, several clean glass slides should be labeled with at least 2 patient identifiers (e.g., name, date of birth, medical record number), and alcohol slide fixative and a container filled with liquid transport medium should be at hand. These will be used to make smears, rinse the needle, and allocate material for special studies if necessary.
Positioning the Patient and Immobilizing the Lesion
The patient is positioned so that he or she is comfortable and the lesion can be palpated and immobilized. The patient should lie on his or her back when feasible; this is a safe position if there is a vasovagal response. Pillows, rolled towels, and foam shapes can be used for support. Changing the patient’s body position can dramatically affect the accessibility of the lesion. Breast masses, for example, are often best appreciated with the patient’s arm raised above the head. Neck nodules easily palpated in the sitting position may seem to disappear when the patient lies on his back. Becoming ambidextrous at FNA allows more flexibility in how the patient is positioned.
If the mass is beneath a band of skeletal muscle like the sternocleidomastoid, position the patient such that the muscle is relaxed and move the muscle aside. In addition to being painful, passing a needle through skeletal muscle clogs the needle with skeletal muscle.
The exact methodology for stabilizing and immobilizing the mass varies by the site, the size of the mass, and the characteristics of the aspirator’s hands.
Once the method of immobilization and the needle trajectory have been determined, the skin is cleaned with an alcohol swab and local anesthesia injected (if desired). Buffered lidocaine solution tends to be less painful than unbuffered.9 Local anesthetic is advisable if the mass is tender to palpation or if the procedure involves a sensitive site like the nipple/areola. It is best not to inject so much local anesthetic that excessive skin swelling obscures the mass. This is particularly true with smaller nodules. Local anesthesia requires a few minutes to take effect.
Sampling the Lesion
Once the mass is fixed with the non-aspirating hand, the skin is cleaned with an alcohol swab at the planned needle entry site. The loosened needle cap is removed and the aspirating hand stabilized by resting the syringe barrel against the thumb or forefinger of the non-aspirating hand. This guards against any physiologic hand tremor and ensures precise needle placement, but is not be needed after insertion of the needle. The needle is inserted into the lesion and the syringe plunger pulled back to generate several cubic centimeters of vacuum. The vacuum is maintained until the needle is removed from the patient. With a straight wrist, the needle is moved back and forth quickly and repeatedly in a sawing motion (“excursions”) for a dwell time no longer than 15-20 seconds (approximately 40-60 excursions) along the original needle trajectory, alternately advancing into the mass and withdrawing to a superficial location without exiting the patient. Slower needle action will yield less material. A shorter dwell time (2 to 5 seconds) is recommended for vascular lesions like thyroid nodules.10 Some practitioners also rotate the hand in a clockwise or counterclockwise fashion while it is moved within the lesion to achieve a “coring” effect, but this is not necessary. Each time the needle advances into the lesion, its cutting tip dislodges small tissue fragments; this cutting action is essential for a successful FNA. Negative pressure alone without needle movement will not procure enough tissue for diagnosis in solid lesions.1 The vacuum in the syringe helps conduct the tissue fragments into the needle shaft and hub. A slight acceleration of the needle as it advances into the mass enhances the cutting action of the needle tip. Keeping the needle tip within the mass avoids diluting the specimen with adjacent non-lesional tissue. Material can be seen accumulating in the needle hub, although absence of visible material does not signify an inadequate sample. If blood is rapidly entering the hub, withdraw the needle immediately, especially in a vascular site like the thyroid gland.
There are nuances to the technique for different sites and types of lesions. Sampling with thinner needles (25g or 27g) is preferred for vascular organs like the thyroid, as well as for fibrous lesions like a fibroadenoma of the breast.3, 10, 11 When sampling a sclerotic lesion, the needle should be moved more vigorously.
To sample more of the mass with one needle pass, withdraw the needle tip to a superficial location while maintaining vacuum, then redirect it to a different area by changing the angle of entry. “Fanning” allows for sampling of a larger area: after each excursion, when the needle tip is in a superficial location, change its angle of entry slightly until the entire region of interest is sampled. Avoid changing direction when the needle is deep in the lesion. This results in tissue tearing and hemorrhage, which compromises the diagnostic yield of subsequent passes.
Remove the needle from the patient after the last excursion is completed or when material or blood is visible in the needle hub, which can occur in less than 15 seconds. Withdraw the needle from the patient in a controlled manner and only after you have released the vacuum in the syringe. Failure to release suction before withdrawing the needle from the patient pulls the aspirated material into the barrel of the syringe, making it difficult to expel for smear preparation. Pressure to the site is applied immediately with gauze to minimize bleeding. The patient or an assistant should perform this step, because the aspirator needs to prepare the sample immediately.
An FNA procedure typically involves inserting the needle into the mass 2 or 3 times (“passes”) to obtain several samples. The center of the mass is often sampled on the first needle pass with the needle approximately perpendicular to the skin. Other areas of the mass are sampled on subsequent passes, especially if the initial material is necrotic, cystic, or otherwise non-diagnostic. Sampling the mass along its long axis tends to yield more cellular specimens compared with moving the needle along the short axis.12
VARIATIONS ON BIOPSY TECHNIQUE
Vacuum suction can be created without a syringe holder in one of two ways. The syringe barrel can be gripped with all the fingers of the aspirating hand while the thumb pulls back the plunger. Alternatively, the plunger is gripped with all fingers and the barrel pushed by the index finger. The latter method requires more finger strength and is a bit easier for those with longer fingers.
The FNA biopsy can be performed without suction (the Zajdela or “French” technique),19 using only the needle or the needle attached to a syringe with the plunger either pulled out part way or completely removed. The needle or syringe is held like a pencil in the aspirating hand, which is stabilized by resting the wrist on an available fixed surface (e.g., the examining table or, with his/her permission, the patient). Without suction, the amount of material harvested is generally lower, but the preparations are also less bloody. This method places the aspirating hand closer to the sampled mass, which allows greater ability to “feel with the needle” and more needle control. It is especially useful when sampling very small or very vascular lesions.
All of the above sampling methods may also be performed using ultrasound guidance if the lesion is difficult to palpate or image-documentation of needle placement inside the lesion is desired. The ultrasound probe is held in place by either the non-aspirating hand or by an assistant. The needle may be positioned parallel or perpendicular to the length of the ultrasound beam.
“Feeling with the Needle”
Learning to feel with the needle is an important skill. Cancer is often described as “gritty to needle,” like pushing a needle through the flesh of a pear. Aspirating a fibroadenoma of the breast feels like pushing a needle into a rubber stopper or leather. Fat is “soft to needle,” meaning that the needle encounters minimal or no resistance. Calcifications are rock-hard. The closer the aspirating hand is to the mass, the more tactile information is perceived. Feeling with the needle allows detection of differences within a mass and is useful for sensing whether the needle has entered or exited the mass. This information is very useful for clinical-pathologic correlation.
POST-PROCEDURE INFORMATION FOR THE PATIENT
Evaluate the patient before allowing him or her to stand up. If the patient is dizzy or light-headed, have the patient remain supine longer. Apply firm pressure for several minutes to the procedure site to minimize bruising, longer if the patient has a history of coagulopathy or is on blood thinners.
Your parting words to the patient might go something like this: “The final report should be ready in a couple days and the results will go to Dr. Jones, who will then contact you. The report may take a little longer if additional tests are needed. You can go about your daily routine, including showering and swimming. You might feel some soreness; this should go away in a few days. In the meantime, apply an ice pack and/or take Tylenol if needed. Also, the lump might seem bigger than it was; this is due to some swelling from the procedure. The lump will return to its original size within a week or so. If you see signs of infection such as fever, or redness/pain/discharge at the biopsy site, call Dr. Jones. He knows you have had this procedure and will know what to do.”
MANAGEMENT OF ADVERSE AND UNEXPECTED EVENTS
If the needle enters an artery, bright red blood shoots into the syringe barrel in a pulsatile fashion. The needle is immediately removed and firm pressure applied to the site for several minutes. The blood in the syringe is best submitted for cell block preparation.
If the patient experiences unusual symptoms (e.g., radiating tingling or pain), the procedure is stopped and the needle removed. Any specimen already procured is prepared, and the patient is observed. It is advisable to wait until symptoms have resolved before performing additional passes. If the patient experiences extreme pain (e.g., schwannomas are typically painful when aspirated) the procedure should be abandoned. Repeat sampling under sedation/anesthesia should be considered if tissue biopsy is still desired.
Familiar with emergency procedures (e.g., calling a code) is advisable in the very unusual event that a patient has a change in heart rate or experiences difficulty breathing.
If the patient moves unexpectedly during the procedure, the risk of a needle stick injury can be reduced by removing the needle from the patient only when it is safe to do so.
Needle stick injuries must be documented and appropriate medical care sought immediately as per institutional guidelines. Many institutions have a 24-hour hotline and/or dedicated beeper for handling needle stick injuries.
Complications of FNA and their resolution should also be documented in the medical record.
REFERENCES
1. Martin HE, Ellis EB. Biopsy by Needle Puncture and Aspiration. Ann Surg 1930;92(2): 169-81.
2. Ljung BM, Drejet A, Chiampi N, et al. Diagnostic accuracy of fine-needle aspiration biopsy is determined by physician training in sampling technique. Cancer 2001;93(4): 263-8.
3. Guidelines of the Papanicolaou Society of Cytopathology for fine-needle aspiration procedure and reporting. The Papanicolaou Society of Cytopathology Task Force on Standards of Practice. Diagn Cytopathol 1997;17(4): 239-47.
4. Duston MA, Skinner M, Shirahama T, Cohen AS. Diagnosis of amyloidosis by abdominal fat aspiration. Analysis of four years' experience. Am J Med 1987;82(3): 412-4.
5. Libbey CA, Skinner M, Cohen AS. Use of abdominal fat tissue aspirate in the diagnosis of systemic amyloidosis. Arch Intern Med 1983;143(8): 1549-52.
6. Guy CD, Jones CK. Abdominal fat pad aspiration biopsy for tissue confirmation of systemic amyloidosis: specificity, positive predictive value, and diagnostic pitfalls. Diagn Cytopathol 2001;24(3): 181-5.
7. Ansari-Lari MA, Ali SZ. Fine-needle aspiration of abdominal fat pad for amyloid detection: a clinically useful test? Diagn Cytopathol 2004;30(3): 178-81.
8. Westermark P, Stenkvist B. A new method for the diagnosis of systemic amyloidosis. Arch Intern Med 1973;132(4): 522-3.
9. Orlinsky M, Hudson C, Chan L, Deslauriers R. Pain comparison of unbuffered versus buffered lidocaine in local wound infiltration. J Emerg Med 1992;10(4): 411-5.
10. Pitman MB, Abele J, Ali SZ, et al. Techniques for thyroid FNA: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol 2008;36(6): 407-24.
11. Zavala DC, Schoell JE. Ultrathin needle aspiration of the lung in infectious and malignant disease. Am Rev Respir Dis 1981;123(1): 125-31.
12. Kreula J, Bondestam S, Virkkunen P. Sample size in fine needle aspiration biopsy. Br J Surg 1989;76(12): 1270-2.
13. Hoda RS. Non-gynecologic cytology on liquid-based preparations: A morphologic review of facts and artifacts. Diagn Cytopathol 2007;35(10): 621-34.
14. Afify AM, Liu J, Al-Khafaji BM. Cytologic artifacts and pitfalls of thyroid fine-needle aspiration using ThinPrep: a comparative retrospective review. Cancer 2001;93(3): 179-86.
15. Parfitt JR, McLachlin CM, Weir MM. Comparison of ThinPrep and conventional smears in salivary gland fine-needle aspiration biopsies. Cancer 2007;111(2): 123-9.
16. Dey P, Luthra UK, George J, Zuhairy F, George SS, Haji BI. Comparison of ThinPrep and conventional preparations on fine needle aspiration cytology material. Acta Cytol 2000;44(1): 46-50.
17. Tulecke MA, Wang HH. ThinPrep for cytologic evaluation of follicular thyroid lesions: correlation with histologic findings. Diagn Cytopathol 2004;30(1): 7-13.
18. Kulkarni MB, Prabhudesai NM, Desai SB, Borges AM. Scrape cell-block technique for fine needle aspiration cytology smears. Cytopathology 2000;11(3): 179-84.
19. Zajdela A, Zillhardt P, Voillemot N. Cytological diagnosis by fine needle sampling without aspiration. Cancer 1987;59(6): 1201-5.
20. Jadusingh IH. Fine needle aspiration biopsy of superficial sites in patients with hemostatic defects. Acta Cytol 1996;40(3): 472-4.
21. Alkan S, Kosar AT, Erdurak SC, Dadas B. Transient vocal cord paralysis following ultrasound-guided fine-needle aspiration biopsy for a thyroid nodule. J Otolaryngol Head Neck Surg 2009;38(1): E14-5.
22. Mayall F, Chang B. Pneumothorax following fine needle aspiration (FNA) of small axillary lymph node. Cytopathology 1998;9(4): 283-5.
23. Priola AM, Priola SM, Cataldi A, et al. Diagnostic accuracy and complication rate of CT-guided fine needle aspiration biopsy of lung lesions: a study based on the experience of the cytopathologist. Acta Radiol 2010;51(5): 527-33.
24. Catania S, Boccato P, Bono A, et al. Pneumothorax: a rare complication of fine needle aspiration of the breast. Acta Cytol 1989;33(1): 140.
25. Gateley CA, Maddox PR, Mansel RE. Pneumothorax: a complication of fine needle aspiration of the breast. Br Med J 1991;303(6803): 627-8.
26. Goodson WH, 3rd, Mailman R, Miller TR. Three year follow-up of benign fine-needle aspiration biopsies of the breast. Am J Surg 1987;154(1): 58-61.
27. Kaufman Z, Shpitz B, Shapiro M, Dinbar A. Pneumothorax. A complication of fine needle aspiration of breast tumors. Acta Cytol 1994;38(5): 737-8.
28. Ustymowicz A, Guzinska-Ustymowicz K, Kordecki K, Lewszuk A, Krejza J. Carotid artery dissection - an important complication after fine-needle aspiration biopsy. Med Sci Monit 2004;10 Suppl 3: 120-2.
29. Engzell U, Franzen S, Zajicek J. Aspiration biopsy of tumors of the neck. II. Cytologic findings in 13 cases of carotid body tumor. Acta Cytol 1971;15(1): 25-30.
30. Llovet JM, Vilana R, Bru C, et al. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology 2001;33(5): 1124-9.
31. Maturen KE, Nghiem HV, Marrero JA, et al. Lack of tumor seeding of hepatocellular carcinoma after percutaneous needle biopsy using coaxial cutting needle technique. AJR Am J Roentgenol 2006;187(5): 1184-7.
32. Smith EH. Complications of percutaneous abdominal fine-needle biopsy. Review. Radiology 1991;178(1): 253-8.
33. Wu M, Burstein DE. Fine needle aspiration. Cancer Invest 2004;22(4): 620-8.
34. Roussel F, Nouvet G. Evaluation of large-needle biopsy for the diagnosis of cancer. Acta Cytol 1995;39(3): 449-52.
35. Liebens F, Carly B, Cusumano P, et al. Breast cancer seeding associated with core needle biopsies: a systematic review. Maturitas 2009;62(2): 113-23.
36. Rodgers MS, Collinson R, Desai S, Stubbs RS, McCall JL. Risk of dissemination with biopsy of colorectal liver metastases. Dis Colon Rectum 2003;46(4): 454-8; discussion 58-9.
37. Hix WR. Chest wall recurrence of lung cancer after transthoracic fine needle aspiration biopsy. Ann Thorac Surg 1990;50(6): 1020-1.
38. Metcalfe MS, Bridgewater FH, Mullin EJ, Maddern GJ. Useless and dangerous--fine needle aspiration of hepatic colorectal metastases. Br Med J 2004;328(7438): 507-8.
39. Navarro F, Taourel P, Michel J, et al. Diaphragmatic and subcutaneous seeding of hepatocellular carcinoma following fine-needle aspiration biopsy. Liver 1998;18(4): 251-4.
40. Lee KC, Chan JK, Ho LC. Histologic changes in the breast after fine-needle aspiration. Am J Surg Pathol 1994;18(10): 1039-47.
41. Douville NJ, Bradford CR. Comparison of ultrasound-guided core biopsy versus fine-needle aspiration biopsy in the evaluation of salivary gland lesions. Head Neck 2012; 00: 000–000.
42. Kumar N, Sharma P, Jain S. Needle stick injuries during fine needle aspiration procedure: Frequency, causes and knowledge, attitude and practices of cytopathologists. J Cytol 2011;28(2): 49-53.
Additional Resource
Video demonstration of FNA Technique by Dr. Britt-Marie Ljung, Papanicolaou Society website (http://www.papsociety.org/fna.html ).
PREPARING THE SAMPLE
Making Smears
To prepare smears, the needle is detached from the syringe, the plunger drawn back to fill the syringe barrel with air, and the needle reattached. This is a very important step but one that carries the risk of a needle stick. Although the risk is lower with Luer-Lok syringes, attaching and detaching needles from these syringes takes more effort and time. One way to minimize this is to detach and reattach the needle by clamping the hub with a hemostat.
To expel the material, the plunger is depressed back into the syringe. It is advisable to hold the needle hub securely on the syringe during this step to avoid having the needle detach and fly away under the increased pressure. Touching the needle tip, bevel-down, to the glass slide minimizes spraying of the material. Spraying causes the sample to dry, which is counter-productive if one is planning to wet-fix the slide in alcohol, and it may aerosolize infectious particles. Aspirated material can also be squirted directly into a liquid medium.
If the amount of material is small, one smear is made. If there is more material or blood, more smears should be made. Because drying begins the moment material is expressed onto a slide, it is important that smears be made as quickly as possible.
The “one-smear” method. The slide with the expelled material is held in one hand (usually the non-dominant hand), frosted side up. The thumb rests on the frosted section, and the remaining fingers are placed along the back side of the slide. The dominant hand holds a clean slide (the “spreader” slide) at a 90° angle to the first slide. The lower edge of the spreader slide is brought into contact with the first slide, and the leading edge of the spreader slide is gently lowered (rotated) until the slide contacts the first slide and compresses the expelled material. Maintaining contact between the slides, the spreader (top) slide is smoothly pulled over the material to distribute the material evenly. If done correctly, the smeared material forms an oval shape on the slide. The spreader slide contains minimal material and, for this reason, is discarded in some laboratories.
The “two-smear” method. The slide with the specimen is oriented horizontally with the frosted side up, and a second slide is placed over it frosted side down. The two slides are gently pressed together until the material is flattened between them, and the slides are pulled apart to make two identical smears. It is more difficult to maintain the parallel orientation of the slides with this method, and there is a higher likelihood of preparation artifact (e.g., uneven distribution of material on the slide or scraping of material by the slide edge).
Splitting Material for Multiple Smears
An abundant harvest can be divided to make two or more smears.
Method 1. This process is similar to the “one-smear” method. The slide with the expelled material is held in one hand (usually the non-dominant hand), frosted side up. The slide is positioned at about a 45° angle from vertical with the thumb on the frosted area, and all other fingers along the back of the slide. With the other (dominant) hand, the spreader slide is held at 90° to above over the first slide. The lower edge of the spreader slide is brought into contact with the first slide, and its leading edge is lowered (rotated) until the slide contacts and slightly compresses the expelled material. Some material will transfer to the spreader slide. The spreader slide is then separated from the first slide. The first slide is set aside and a clean slide picked up while held in the same newspaper-reading position below the spreader slide. A smear with this second slide is made in the same way. This process can be repeated to make multiple smears.
Method 2. Instead of expelling all the aspirated material onto one slide, a small drop of material is expressed onto multiple slides. This method requires more control of the plunger. With a single spreader slide, a smear is made on each slide.
Fixing the Smears
Generally, at least one alcohol-fixed and one air-dried smear should be made from each needle pass. The advantages and disadvantages are shown in Table 8.2. Alcohol fixation provides better nuclear detail, whereas air-drying allows better visualization of cytoplasmic quality and extracellular material like mucin and cartilage. For rapid evaluation, an air-dried smear can be stained with a Romanowsky-type stain and examined without a cover slip. Although more time-consuming, an alcohol-fixed slide can be stained with either a rapid Papanicolaou or toluidine blue stain and examined with a cover slip. Air-dried smears should be dried quickly to avoid slow-dry artifacts. Thick, wet slides are often fanned to expedite drying. When preparing alcohol-fixed smears, the slide should be immersed in 95% ethanol or sprayed with an alcohol-based fixative without delay after the smear is made to avoid air-dry artifacts.
Handling Cystic Masses
If the mass is cystic, fluid fills the syringe barrel under negative pressure. As much fluid as possible should be aspirated; applying pressure on the mass can assist with this. The fluid can be discarded if appropriate (e.g., clear fluid from a breast cyst) or expelled directly into a container for subsequent processing. The patient is re-examined, and any residual mass sampled on a subsequent pass. Some cystic lesions are best aspirated under ultrasound guidance, as this allows the aspirator to target the solid area of a heterogeneous mass.
Retrieving Material from the Needle Hub
Some aspirated material may remain in the needle hub after attempts at expulsion onto glass slides. To extract this material, the needle is detached from the syringe and its tip pushed into the rubber top of a blood draw tube or a needle safety device such that the needle tip is not exposed.The needle hub is placed in the middle of a clean glass slide. In a coordinated manner, the glass tube is rocked back and forth with one hand, while the other hand flicks the needle hub. This technique takes some practice. Alternatively, with the needle tip firmly in the rubber top or safety device, the needle is inverted and its hub tapped against a clean glass slide to dislodge tissue fragments.
In most cases, an FNA should not be performed on a given site more than 3 to 4 times at one clinic visit. Repeated biopsies increase tissue hemorrhage, reducing diagnostic yield. It is better to have the patient return in several days for a repeat FNA.
Rinsing the Needle and Reserving Material for Ancillary Studies
To harvest as much of the sample as possible, the needle should be rinsed using a liquid medium. The needle tip is placed in a container filled with the medium; a small amount of liquid is drawn into the syringe by pulling back on the plunger, then expelled back into the container by pushing in the plunger. This is repeated several times. Excessive force should be avoided as this may mechanically damage the cells. Note that smears should be made before the needle is rinsed to avoid drying artifacts.
The needle can be rinsed with saline, a culture medium like RPMI, or a commercial hemolytic transport solution (e.g., CytoLyt®, CytoRich Red®) for cell block and/or liquid-based preparations (e.g., ThinPrep, SurePath). For routine assessment, the accuracy of thin-layer preparations is comparable to that obtained with smears. One should be aware of slight differences in the cytologic appearances of cells in thin-layer preparations, however, such as smaller and more three-dimensional cell clusters, smaller and darker nuclei, and a cleaner background.13-17
The choice of medium for sample collection depends also on what ancillary studies are needed for diagnosis. The most versatile sample is the needle rinse in saline or culture medium like RPMI; such sample can be submitted for flow cytometric assessment of lymphoid markers in the case of a suspected lymphoma, or for a karyotype in the case of a suspected soft tissue neoplasm. On the other hand, some techniques like fluorescence in situ hybridization (FISH) and molecular assays that amplify DNA are very robust and can be performed successfully regardless of the cytologic substrate, whether a smear, cell block, or liquid-based preparation, so long as certain cellular adequacy criteria are met.
Making a Cell Block from a Smear
If an adequate cell block was not obtained after sedimenting the needle rinse sample, the material on a smear, even if previously stained, can be re-purposed into a cell block. A cellular smear containing large tissue particles is best for this purpose. The slide is soaked in xylene until the coverslip falls off on its own. (This may take several days.) The material is scraped off the slide with a scalpel blade onto lens paper or a sponge and submitted for usual processing as a paraffin-embedded cell block.18
References
1. Martin HE, Ellis EB. Biopsy by Needle Puncture and Aspiration. Ann Surg 1930;92(2): 169-81.
2. Ljung BM, Drejet A, Chiampi N, et al. Diagnostic accuracy of fine-needle aspiration biopsy is determined by physician training in sampling technique. Cancer 2001;93(4): 263-8.
3. Guidelines of the Papanicolaou Society of Cytopathology for fine-needle aspiration procedure and reporting. The Papanicolaou Society of Cytopathology Task Force on Standards of Practice. Diagn Cytopathol 1997;17(4): 239-47.
4. Duston MA, Skinner M, Shirahama T, Cohen AS. Diagnosis of amyloidosis by abdominal fat aspiration. Analysis of four years' experience. Am J Med 1987;82(3): 412-4.
5. Libbey CA, Skinner M, Cohen AS. Use of abdominal fat tissue aspirate in the diagnosis of systemic amyloidosis. Arch Intern Med 1983;143(8): 1549-52.
6. Guy CD, Jones CK. Abdominal fat pad aspiration biopsy for tissue confirmation of systemic amyloidosis: specificity, positive predictive value, and diagnostic pitfalls. Diagn Cytopathol 2001;24(3): 181-5.
7. Ansari-Lari MA, Ali SZ. Fine-needle aspiration of abdominal fat pad for amyloid detection: a clinically useful test? Diagn Cytopathol 2004;30(3): 178-81.
8. Westermark P, Stenkvist B. A new method for the diagnosis of systemic amyloidosis. Arch Intern Med 1973;132(4): 522-3.
9. Orlinsky M, Hudson C, Chan L, Deslauriers R. Pain comparison of unbuffered versus buffered lidocaine in local wound infiltration. J Emerg Med 1992;10(4): 411-5.
10. Pitman MB, Abele J, Ali SZ, et al. Techniques for thyroid FNA: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol 2008;36(6): 407-24.
11. Zavala DC, Schoell JE. Ultrathin needle aspiration of the lung in infectious and malignant disease. Am Rev Respir Dis 1981;123(1): 125-31.
12. Kreula J, Bondestam S, Virkkunen P. Sample size in fine needle aspiration biopsy. Br J Surg 1989;76(12): 1270-2.
13. Hoda RS. Non-gynecologic cytology on liquid-based preparations: A morphologic review of facts and artifacts. Diagn Cytopathol 2007;35(10): 621-34.
14. Afify AM, Liu J, Al-Khafaji BM. Cytologic artifacts and pitfalls of thyroid fine-needle aspiration using ThinPrep: a comparative retrospective review. Cancer 2001;93(3): 179-86.
15. Parfitt JR, McLachlin CM, Weir MM. Comparison of ThinPrep and conventional smears in salivary gland fine-needle aspiration biopsies. Cancer 2007;111(2): 123-9.
16. Dey P, Luthra UK, George J, Zuhairy F, George SS, Haji BI. Comparison of ThinPrep and conventional preparations on fine needle aspiration cytology material. Acta Cytol 2000;44(1): 46-50.
17. Tulecke MA, Wang HH. ThinPrep for cytologic evaluation of follicular thyroid lesions: correlation with histologic findings. Diagn Cytopathol 2004;30(1): 7-13.
18. Kulkarni MB, Prabhudesai NM, Desai SB, Borges AM. Scrape cell-block technique for fine needle aspiration cytology smears. Cytopathology 2000;11(3): 179-84.
19. Zajdela A, Zillhardt P, Voillemot N. Cytological diagnosis by fine needle sampling without aspiration. Cancer 1987;59(6): 1201-5.
20. Jadusingh IH. Fine needle aspiration biopsy of superficial sites in patients with hemostatic defects. Acta Cytol 1996;40(3): 472-4.
21. Alkan S, Kosar AT, Erdurak SC, Dadas B. Transient vocal cord paralysis following ultrasound-guided fine-needle aspiration biopsy for a thyroid nodule. J Otolaryngol Head Neck Surg 2009;38(1): E14-5.
22. Mayall F, Chang B. Pneumothorax following fine needle aspiration (FNA) of small axillary lymph node. Cytopathology 1998;9(4): 283-5.
23. Priola AM, Priola SM, Cataldi A, et al. Diagnostic accuracy and complication rate of CT-guided fine needle aspiration biopsy of lung lesions: a study based on the experience of the cytopathologist. Acta Radiol 2010;51(5): 527-33.
24. Catania S, Boccato P, Bono A, et al. Pneumothorax: a rare complication of fine needle aspiration of the breast. Acta Cytol 1989;33(1): 140.
25. Gateley CA, Maddox PR, Mansel RE. Pneumothorax: a complication of fine needle aspiration of the breast. Br Med J 1991;303(6803): 627-8.
26. Goodson WH, 3rd, Mailman R, Miller TR. Three year follow-up of benign fine-needle aspiration biopsies of the breast. Am J Surg 1987;154(1): 58-61.
27. Kaufman Z, Shpitz B, Shapiro M, Dinbar A. Pneumothorax. A complication of fine needle aspiration of breast tumors. Acta Cytol 1994;38(5): 737-8.
28. Ustymowicz A, Guzinska-Ustymowicz K, Kordecki K, Lewszuk A, Krejza J. Carotid artery dissection - an important complication after fine-needle aspiration biopsy. Med Sci Monit 2004;10 Suppl 3: 120-2.
29. Engzell U, Franzen S, Zajicek J. Aspiration biopsy of tumors of the neck. II. Cytologic findings in 13 cases of carotid body tumor. Acta Cytol 1971;15(1): 25-30.
30. Llovet JM, Vilana R, Bru C, et al. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology 2001;33(5): 1124-9.
31. Maturen KE, Nghiem HV, Marrero JA, et al. Lack of tumor seeding of hepatocellular carcinoma after percutaneous needle biopsy using coaxial cutting needle technique. AJR Am J Roentgenol 2006;187(5): 1184-7.
32. Smith EH. Complications of percutaneous abdominal fine-needle biopsy. Review. Radiology 1991;178(1): 253-8.
33. Wu M, Burstein DE. Fine needle aspiration. Cancer Invest 2004;22(4): 620-8.
34. Roussel F, Nouvet G. Evaluation of large-needle biopsy for the diagnosis of cancer. Acta Cytol 1995;39(3): 449-52.
35. Liebens F, Carly B, Cusumano P, et al. Breast cancer seeding associated with core needle biopsies: a systematic review. Maturitas 2009;62(2): 113-23.
36. Rodgers MS, Collinson R, Desai S, Stubbs RS, McCall JL. Risk of dissemination with biopsy of colorectal liver metastases. Dis Colon Rectum 2003;46(4): 454-8; discussion 58-9.
37. Hix WR. Chest wall recurrence of lung cancer after transthoracic fine needle aspiration biopsy. Ann Thorac Surg 1990;50(6): 1020-1.
38. Metcalfe MS, Bridgewater FH, Mullin EJ, Maddern GJ. Useless and dangerous--fine needle aspiration of hepatic colorectal metastases. Br Med J 2004;328(7438): 507-8.
39. Navarro F, Taourel P, Michel J, et al. Diaphragmatic and subcutaneous seeding of hepatocellular carcinoma following fine-needle aspiration biopsy. Liver 1998;18(4): 251-4.
40. Lee KC, Chan JK, Ho LC. Histologic changes in the breast after fine-needle aspiration. Am J Surg Pathol 1994;18(10): 1039-47.
41. Douville NJ, Bradford CR. Comparison of ultrasound-guided core biopsy versus fine-needle aspiration biopsy in the evaluation of salivary gland lesions. Head Neck 2012; 00: 000–000.
42. Kumar N, Sharma P, Jain S. Needle stick injuries during fine needle aspiration procedure: Frequency, causes and knowledge, attitude and practices of cytopathologists. J Cytol 2011;28(2): 49-53.
Additional Resource
Video demonstration of FNA Technique by Dr. Britt-Marie Ljung, Papanicolaou Society website (http://www.papsociety.org/fna.html).
Please see separate organ sections for reporting and terminology details.
GOALS/EXPECTATIONS:
What you are expected to do: Pathology trainees will be expected to perform FNA procedures on patients and prepare specimens under supervision of an attending cytopathologist. Residents may perform procedures under supervision of an approved Cytology Fellow after Fellow has demonstrated competency. Trainee performance will be evaluated and documented.
Daily Schedule:
8am-9am: Attend Cytology Consensus Conference (may not happen every morning). Residents should ask the Cytology Fellows to be included in the group text pages.
9am-12pm: Be available and prepare for FNA procedures.* Review and signout with attending your assigned cytology specimens when not on an FNA procedure.
12pm-1pm: Attend Outs sessions or similar.
1pm-5pm: Be available and prepare for FNA procedures.* Review and signout with attending your assigned cytology specimens when not on an FNA procedure.
- The Cytopathology division at MGH runs the Fine Needle Aspiration Biopsy Clinic located on Wang-270. The FNA Clinic sees patients in 45-minute appointments from 9am-noon and 1pm-5pm Monday-Friday (i.e. 9:00am, 9:45 am, 10:30am, etc.). No patients are seen during the noon-1pm lunch hour, or on weekends. Appointments are booked by the Cytopathology Main Desk (Diane White and Bernadette Femino). Introduce yourself to the Main Desk and ask them to page you with updates to the FNA schedule. Prepare for appointments by making sure the FNA Clinic supplies are stocked and reviewing the patient’s history in CAS/LMR prior to patient’s arrival.
- We also occasionally perform FNA on in-patients at bedside. A cart containing a microscope and FNA kit are wheeled to the patient’s room for these procedures. The FNA cart is located in the Cytopathology Prep Lab (Warren-1).
HOW IS THE FNA ROTATION DIFFERENT FROM OTHER PATHOLOGY ROTATIONS?
You will directly interact with patients, talking with them and performing physical exams. You will learn how to obtain tissue samples from patients using a thin needle while they are awake. You will learn multiple methods to prepare tissue samples for evaluation. Unlike in the gross room, no scalpels are used and not all specimens are fixed in formalin. WHAT IS FINE NEEDLE ASPIRATION (FNA)?
FNA is a tissue sampling procedure that uses a thin needle (defined as 22 gauge or thinner). FNA is used to evaluate palpable superficial masses, both solid and cystic. FNA is also used to sample abdominal fat for amyloid testing. The needle is inserted through the skin into the mass to extract cells. The patient is awake during the procedure, but the biopsy site may be numbed with local injection of anesthetic such as lidocaine.
FNA can also be performed under image-guidance (e.g. ultrasound, CT) to evaluate deep-seated, nonpalpable radiologic abnormalities such as in the lungs or liver. FNA done using endoscopy or bronchoscopy to evaluate sites such as pancreas and mediastinal lymph nodes. The patient is often sedated in these settings and the procedures are performed by non-pathologists.
Because the needle used is so much thinner than the ones commonly used for core biopsy (which can be as large as 8 gauge), the tissue obtained in FNAs consists of tiny fragments that often cannot be discerned by the naked eye.
Review “Introduction” video on http://www.papsociety.org/fna.html.
Materials and Supplies
All the equipment needed to perform an FNA (see Table) is small and lightweight enough to be hand-carried in one container. As a result, FNAs may be performed virtually anywhere on demand. The equipment occupies only a small area of table space when laid out for specimen preparation.
Review “Equipment” video on http://www.papsociety.org/fna.html.
FNA Equipment List
1. Syringe holder (10 mL Cameco syringe pistol or equivalent)
2. Disposable sterile 10 mL plastic syringes
3. Disposable sterile needles with transparent hubs (23g and 25g, 1.0 to 1.5 inches long)
4. Alcohol swabs
5. Sterile gauze pads
6. Glass slides with frosted end for labeling
7. Fluid transport medium (e.g., sterile RPMI, saline, formalin)
8. Local anesthesia and needle/syringe to administer (e.g., 2% lidocaine, 27g/1 cc)
9. Alcohol fixative (commercial spray fixative or jar filled with 95% ethanol)
10. Gloves (preferably non-latex)
11. Pen/pencil for labeling slides (e.g., Leica pen, blue marking pencil)
12. Plastic slide holders or slide trays (for transporting slides)
13. Rubber-topped blood-draw tube for “flip” technique.
FNA Procedure for sampling a palpable mass
Steps for a successful fine needle aspiration (FNA):
Decide if an FNA should be performed Consent the patient for the procedure Position the patient and immobilize the lesion Sample the targeted lesion adequately Prepare the sample for evaluation, including appropriate allocation of material for ancillary studies as necessary Provide post-procedure instructions to the patient
Decide if an FNA should be performed
All patients presenting to us for an FNA have been referred by a clinician. Review the patient’s clinical history and relevant imaging studies before seeing the patient. Perform a focused physical examination to confirm that the lesion is indeed palpable and an FNA is clinically appropriate. While feeling the mass with your fingers, take mental notes on its size, shape, and relationship to any significant structures like large blood vessels. This information guides how you will approach sampling the lesion (e.g., the length of needle to use, vascular areas to avoid).
Sometimes the mass will be marked on the patient’s skin by the referring clinician.
If you have trouble finding the mass, the patient may be able to show you where it is.
During the brief examination, take a moment to ask whether the patient has any history of bleeding disorder or if s/he is on blood thinners.
If the lesion is not palpable or cannot be safely sampled, do not perform an FNA. An alternative is to evaluate the patient and perform the FNA procedure using ultrasound guidance. (See “FNA using Ultrasound” Supplement.)
Consent the Patient
Describe the FNA procedure in detail to the patient, including its purpose and potential complications. Allow the patient to ask questions. Confirm the patient’s name, date of birth, and site to be biopsied. Have the patient sign the procedure consent form.
Sample dialogue with a patient for obtaining consent for the FNA procedure:
“Hello Mr. Smith, I am Dr. Ly from the Department of Pathology. I understand that Dr. Jones has sent you here for a fine needle aspiration biopsy of a mass on your arm. Our goal is to determine the diagnosis for the mass. Let me explain what the procedure involves. Feel free to ask questions at any point.
I will use a very thin needle to sample the mass. The needle is the same size or smaller than the ones used for blood draws. I will insert the needle into the mass and move the needle back and forth for about 15-20 seconds. I usually do this 2 or 3 times, which means 2 or 3 separate needle sticks. I may need to do it a 4th time if I think additional material will help us make a diagnosis. We will take a small break after the 1st or 2nd needle stick so I can quickly check how much material we have by looking under a microscope. This is not to make a diagnosis, but rather it is a quantity check to make sure there is enough material to evaluate.
Every patient’s experience with this procedure is different. Most patients feel a pinprick when the needle goes through the skin just like during a blood draw. While the needle is moving back and forth, most patients feel a pulling sensation or dull pressure or soreness. I can inject lidocaine into the skin over the lump to numb the area. It will be an additional needle stick and you may feel a temporary burning sensation that lasts up to 30 seconds. If during the procedure you experience pain that you cannot tolerate, I will stop and take the needle out.
This procedure has a few minor risks that you should be aware of. The most common are bleeding and slight bruising. After each needle stick, my assistant will apply firm pressure with a clean gauze pad to minimize bleeding. Infection is another possible complication, but I clean the skin well with alcohol before each needle stick to minimize the chances of this happening.
Do you understand the purpose of this procedure and the risks involved? Do you agree to having this procedure? If so, please confirm for me your full name and date of birth, and sign this consent form.”
Prepare the Equipment
Universal safety precautions must be observed during the biopsy procedure and while handling the harvested tissue specimen.
You must prepare the tissue sample immediately before it dries/clots. You will make smears from the sample, and may rinse the needle and allocate material for special studies if necessary. Label several clean glass slides with at least 2 patient identifiers (e.g., name, date of birth, medical record number). Open a jar of alcohol slide fixative. Open a sterile test tube and fill it halfway with saline. Open a small jar of formalin. Place these materials on your table workspace.
Load a syringe onto the syringe holder and attach a needle. Commonly, 23g and 25g needles measuring 1.0 to 1.5 inches long are used. Choose the shortest needle that reaches the deepest area of the lesion from the skin. For example, if the bottom edge of the mass is 1 inch beneath the skin, choose a 1 inch needle, not a 1.5 inch needle. Loosen the needle cap and set the equipment down in a convenient location.
Position the Patient and Immobilize the Mass
Position the patient such that s/he is comfortable and the lesion can be palpated and immobilized (i.e. held fixed in place by the fingers of your non-dominant hand so it does not move around while you are sampling it). Ideally, have the patient lie on his back; this is a safe position in case of a vasovagal response and the patient passes out during the procedure. Support the patient’s body with pillows, rolled up sheets/towels, or foam shapes. Changing the patient’s body position can dramatically affect the accessibility of the lesion. Breast lumps are usually best appreciated with the patient’s arm raised above the head. Neck masses easily felt in the sitting position but may completely disappear when the patient lies on his back.
If the mass is beneath a band of skeletal muscle, position the patient such that the muscle is relaxed and pull or push the muscle aside. Inserting a needle through skeletal muscle is painful for patients, and will clog your needle, preventing you from obtaining a good sample.
Plan ahead how you will immobilize the mass – try different configurations with your fingers (of your non-dominant hand) on the mass. Also plan the angle at which your needle will be inserted into the mass. For many lumps, inserting the needle perpendicular to the skin surface is adequate. Clean the skin with an alcohol swab. If local anesthesia is to be given, start by injecting 0.5 cc to 1.0 cc of 2% buffered lidocaine subcutaneously at the biopsy site. Additional lidocaine can be given at any subsequent point if the patient is uncomfortable. Sensitive areas such as the nipple/areola complex should receive more lidocaine up front (e.g. 2 to 3 cc). Allow a few minutes for the local anesthesia to take effect. Caution: With small nodules (e.g. < 5 mm), injecting too much lidocaine can cause enough skin swelling that your fingers can no longer feel the mass distinctly.
For all patients, but particularly those who decline lidocaine, offer your hand and encourage them to squeeze it during the procedure. This helps to alleviate discomfort and pain.
Review “Special Problems,” “Technique,” and “Stabilizing the Instrument” videos on http://www.papsociety.org/fna.html.
Sample the Mass
Review “Needle Movement” video on http://www.papsociety.org/fna.html.
Once the mass is fixed with the non-aspirating hand, clean the skin with an alcohol swab at the planned needle entry site. Remove the loosened needle cap and stabilize the aspirating hand by resting the syringe barrel against the thumb or forefinger of the non-aspirating hand. A steady hand ensures precise needle placement. Push the needle through the skin into the mass in one motion, and pull back the syringe plunger to generate several cc of suction. At this point, it is no longer necessary to stabilize the aspirating hand. Maintain the vacuum until you are just about to remove the needle from the patient. With a straight wrist, quickly move the needle back and forth in a sawing motion (“excursions”) for no longer than 15-20 seconds (approximately 40-60 excursions) along the original needle trajectory. (For vascular lesions such as thyroid, 2 to 5 seconds is recommended.) Make sure the needle does not exit the patient. Each time the needle advances into the lesion, its cutting tip dislodges small tissue fragments; this cutting action is essential for a successful FNA. Slow needle action will yield less material, and no needle movement at all movement will yield a non-diagnostic sample in solid masses. The vacuum in the syringe helps tissue fragments travel up the needle shaft. Keep the needle tip within the mass to avoid diluting the sample with adjacent non-lesional tissue. As you perform your excursions, you may see material accumulating in the needle hub. If blood rapidly enters the hub, withdraw the needle immediately and apply pressure to the site with gauze.
Sampling with thinner needles (25g or 27g) is preferred for vascular organs like the thyroid, and for fibrous lesions like a fibroadenoma of the breast. When sampling a densely fibrotic mass, your excursions should be more forceful.
Review “Sampling of Different Nodule Parts” video on http://www.papsociety.org/fna.html.
An FNA procedure typically involves inserting the needle into the mass 2 or 3 times (“passes”) to obtain several samples. The center of the mass is often sampled on the first needle pass with the needle approximately perpendicular to the skin. You can try other areas of the mass on subsequent passes, especially if the initial material is necrotic, cystic, or non-diagnostic. Sample the mass along its long axis to obtain more cellular specimens.
To sample more than one area within the mass on one needle pass, withdraw the needle tip to a superficial location, then change its angle of entry to enter a different area of the mass. “Fanning” allows for sampling of a larger area: after each excursion, when the needle tip is in a superficial location, change its angle of entry slightly until the entire region of interest is sampled. Avoid changing direction when the needle is deep in the lesion. This results in tissue tearing and hemorrhage, which compromises the diagnostic yield of subsequent passes.
Remove the needle from the patient after the last excursion or when you see material or blood in the hub, which can occur in < 15 seconds. Release the vacuum in the syringe before withdrawing the needle from the patient. Withdrawing the needle from the patient when there is still negative pressure in the syringe will force aspirated material up into the syringe barrel, and it cannot be used to make smears (you can still retrieve it by rinsing the needle as described below). Hold firm pressure to the biopsy site with gauze for several minutes, longer if there is a history of coagulopathy or blood thinners. The patient or an assistant should perform this step, because the aspirator needs to prepare the sample immediately.
Prepare the sample
Make Smears
There are multiple ways of preparing direct smears from the aspirated material retrieved from the needle and needle hub. Because aspirated material will vary in quantity and quality from case to case, it is important to be familiar with several different techniques.
Review “Expulsion onto slide,” ”Flip Technique,” “Basic Smearing Technique,” “Dividing Material,” and “Problem Material” videos on http://www.papsociety.org/fna.html.
Fix the Smears
Generally, at least one alcohol-fixed and one air-dried smear should be made from each needle pass. The advantages of each fixation method are shown below. Air-dried smears should be dried quickly to avoid drying artifacts. When fixing with alcohol, submerge the smear in 95% ethanol immediately after it is made to avoid air-dry artifacts.
For information about making cell blocks from smears, see "Reserving Material for Ancillary Studies" and "Making a Cell Block from a Smear" on our sample preparation page.
| Air-dried Smears | Alcohol-fixed Smears | |
|---|---|---|
| Useful for Rapid Evaluation? | Yes; stain on-site with DiffQuik. | Superior nuclear detail. |
| Yes; stain on-site with Toluidene blue | Rapid on-site evaluation (if a modified Ultrafast Pap stain, toluidine blue, or rapid H&E stain used). | |
| Yes; allows for evaluation of lesions with background material | ||
| Advantages | Enhances cellular pleomorphism. | Superior nuclear detail. |
| Fast and easy to perform. | Less air-drying artifact and enlargement. | |
| Better for evaluating background material and cellular cytoplasm (e.g., mucin, cartilage) | Better for evaluating squamous lesions. | |
| Better for visualizing some organisms such as mycobacteria (“negative images”). |
Assess Specimen Adequacy
Rapid on-site evaluation of smears can be performed. This is the Cytology equivalent of frozen sections.
Choose 1 or 2 cellular slides for immediate evaluation. If air-dried, stain using Diff-Quik and do not coverslip. If alcohol-fixed, stain using Toluidine Blue and coverslip. Evaluate the slides under the microscope and determine whether the quantity and quality of the material present is sufficient for diagnosis. Determine whether obtaining material for ancillary studies will be helpful (e.g. flow cytometry, cell block for IHC).
Diff-Quik Staining Procedure: 5 dips in methanol, 5 dips in DQ-I solution (with eosin), 5 dips in DQ-II solution (with methylene blue), 5 dips in tap water to rinse. Observe the stained material. If it is red/brown/orange, dip again in DQ-II and rinse in tap water. If the material is blue/purple, staining is adequate. Dry back of slide with paper towel and review it under the microscope without coverslip. Background material and cytoplasmic quality are accentuated with this stain. Nuclear membrane irregularities may not be as obvious as on alcohol-fixed material. Cells appear larger and more pleomorphic than if alcohol-fixed.
Toluidine Blue Staining Procedure: Remove slide from jar of alcohol and place on tabletop face-up (cellular material is on top surface). Using eyedropper, place 1-2 drops of Toluidine Blue solution onto slide. Coverslip and remove excess fluid using capillary action by tilting slide onto its edges. After allowing stain to penetrate for 30-60 seconds, dry back of slide and evaluate under microscope. Nuclei will appear dark blue with this stain. Background material and cytoplasmic quality are usually not well-visualized.
Waste Disposal: Discard used tap water and stains into dedicated plastic container using funnel. Container is located under the counter in left cabinet.
How to Handle Cyst Aspirations
If the mass is cystic, you will see fluid filling the syringe barrel under negative pressure. Aspirate as much fluid as possible from the cyst; pushing on the mass can assist with this. The fluid can be discarded if appropriate (e.g., clear fluid from a breast cyst) or deposited directly into a container for subsequent processing. The patient is re-examined, and any residual mass sampled on a subsequent pass.
Avoid performing an FNA on a given site more than 3 to 4 times at one clinic visit. Repeated biopsies increase tissue hemorrhage, reducing diagnostic yield. It is better to have the patient return in several days for a repeat FNA.
Rinse the Needle and Reserve Material for Ancillary Studies
To harvest as much of the sample as possible, rinse the needle into a liquid medium. Place the needle tip in a container of medium and draw a small amount of liquid into the syringe by pulling back on the plunger. Then expel it back into the container by pushing in the plunger. Gently repeat this a few times. Excessive force may mechanically damage cells.
The needle can be rinsed with several different media. We use saline, formalin, and/or SurePath fixative. The medium you choose will depend in part on what ancillary studies you anticipate will be needed. In our lab, the most versatile sample is the needle rinse in saline.
| Type of Preparation | Ancillary Test | |||||
|---|---|---|---|---|---|---|
| Flow Cytometry | Cell Block | Cytogenetics (Karyotyping) | FISH | DNA-based Molecular Test | Immuno-histochemistry | |
| Smear | No | Yes | No | Yes | Yes | Yes |
| Formalin-fixed cell block | No | Yes | No | Yes | Yes | Yes |
| ThinPrep or SurePath fixative | No | Yes | No | Yes | Yes | Yes |
| RPMI/saline | Yes | Yes | Yes | Yes | Yes | Yes |
| needle rinse |
Post-Procedure Information for The Patient
Evaluate the patient before allowing her to stand up. If she is dizzy or light-headed, have the patient remain supine longer.
Give the patient a copy of “Fine Needle Aspiration Biopsy; Post-Procedure Instructions for Patients.”
Example of parting words to the patient:
“The final report should be ready in a few days and the results will go directly to Dr. Jones, who will then contact you. The report may take a little longer if additional tests are needed. Here are some instructions and information for you to take with you. You can go about your daily routine, including showering and swimming. If you have a small amount of bleeding later today, apply an ice pack for 20 minutes to help stop the bleeding. You might feel some soreness; this will go away in a few days. In the meantime, take Tylenol if you are uncomfortable. Also, the lump might seem bigger than it was before; this is due to some swelling from the procedure. The lump will return to its original size within a week or so. If you see signs of infection such as fever and redness/pain/discharge at the biopsy site, or if there is persistent bleeding, call Dr. Jones’ office.
Variations on Biopsy Technique
For information on "feeling with the needle", and further info about technique variations and preparing/procuring the specimen see our procuring the specimen page.
Zajdela Technique
The FNA biopsy can be performed without suction (the Zajdela or “French” technique) using only the needle or the needle attached to a syringe, with the plunger either pulled out part way or completely removed. The needle hub or syringe is held like a pen in the aspirating hand, which is stabilized by resting the wrist on an available fixed surface (e.g., exam table, a part of the patient’s body). Without suction, the amount of material harvested is typically lower, but the preparations are also less bloody. This method places the aspirating hand closer to the sampled mass, which allows more needle control and superior tactile perception of the tissues penetrated by the needle. It is especially useful when sampling very small or very vascular lesions.
Review “Zajdela’s Technique” video on http://www.papsociety.org/fna.html.
Sampling two or more distinct areas in a mass in one needle pass
More than one area in a mass may be sampled using just one needle pass. Sample the first area as usual. Then withdraw the needle tip to a superficial location (e.g. dermis) while maintaining vacuum and without exiting the patient. Change the needle’s angle of entry to redirect it to a different area and sample by performing excursions. Repeat as required.
Sampling a large region in a mass in one needle pass
A larger area in a mass may be sampled using just one needle pass using a technique called “fanning.” After each excursion when the needle tip is in a superficial location, change its angle of entry slightly until the entire region of interest is sampled. Changing the needle direction while the needle is deep in the lesion is to be avoided as it can cause tissue tearing and hemorrhage, which will then compromise the diagnostic yield of subsequent passes.
How can I practice sampling masses by FNA?
You can practice sampling with the syringe holder, with or without ultrasound, using phantom body parts. We have a simulated neck and a simulated breast in the FNA Clinic for this purpose.
You can practice French technique for sampling by taking home a syringe and a few needles. Some suggested surrogate targets: fruits, vegetables, raw meat.
POTENTIAL Complications
Minor pain, bleeding, and bruising are most common. A significant hematoma develops occasionally. Malignant lesions tend to bleed more than benign ones. Stop any bleeding by applying firm pressure to the biopsy site for several minutes. This is sufficient even in patients with coagulopathy. Some patients experience a vasovagal response; prevent falls in case of fainting by positioning the patient supine on the exam table.
Pneumothorax can rarely occur when aspirating a lesion near the chest wall. The patient will experience immediate chest and/or shoulder pain. The risk is greatest in thin patients. Choose a needle trajectory that is tangential to the rib cage to reduce the risk.
Table 8.4 frequency of Pneumothorax as a Complication of Fine Needle Aspiration (FNA) of Superficial (Palpable) Lesions
| Authors | Cases of pneumothorax | Total FNAs | Incidence of pneumothorax (%) |
|---|---|---|---|
| Catania et al24 | 13 | 74,000 | 1:5693 (0.018) |
| Gateley et al25 | 7 | not given | 1:1000 (0.10) |
| Goodson et al26 | 1 | 285 | 1:285 (0.35) |
| Kaufman et al27 | 4 | 1666 | 1:417 (0.24) |
Needle stick injury to the aspirator may occur whenever the needle tip is exposed. Avoid such injuries with vigilance and attention to the location of the needle tip at all times. Always exercise universal precautions.
Management of Adverse and Unexpected Events
1) If the needle enters an artery, bright red blood shoots into the syringe barrel in a pulsatile fashion. Immediately remove the needle from the patient and apply firm pressure with gauze to the site for several minutes. Submit the blood in the syringe for cell block preparation.
2) If the patient experiences neurologic symptoms (e.g., radiating pain or tingling), stop the procedure and remove the needle. Prepare any sample already procured and observe the patient. This condition is transient. Wait for the symptoms to resolve before attempting additional sampling. If the patient experiences extreme/intolerable pain (e.g., schwannomas are quite painful when aspirated), abort the procedure and suggest repeat sampling with sedation.
3) If the patient has a change in heart rate or experiences difficulty breathing, call a code (x6-3333).
4) If the patient moves unexpectedly during the procedure, you may accidentally stick yourself with the needle. Reduce the risk of a needle stick injury by keeping the needle tip inside the patient and removing the needle from the patient only when it is safe to do so.
5) If you are stuck by a contaminated needle: a) stay calm, b) wash injury with soap and water, c) pat dry and apply bandage, d) apply pressure through bandage if still bleeding, and e) call Occupational Health for instructions (x6-2217).
6) Document any patient complications of FNA and their resolution in the medical record.
Where to Drop off specimens and FoRMS for ancillary studies
Cytology Requisition- Cytology Lab (yellow form)
Cell Block- Cytology Lab (same yellow form as Cytology Requisition)
Flow Cytometry- Cytology Lab (fill out separate form available in Lab)
Microbiology- Cytology Lab (fill out separate form available in Lab)
Cytogenetics- Frozen section lab (Blake 3). Specimen must be put in media available from grossing area.
EM- Currently no separate EM form used. Obtain a small vial of glutaraldehyde from the frozen section lab (Blake 3) in freezer compartment of small fridge. Allow the media to thaw 5-10 min at room temperature, and then deposit the specimen in the media. Photocopy the Cytology Requisition, place the copy in a specimen bag with the sample, and leave the bag in a small white container labeled “EM for Warren 5” on shelf inside the fridge door.
ADDITIONAL forms
Completed FNA Template - leave with Souad Mouni for typing (Warren 105).
CYTOLOGY FELLOWS ONLY: INSTRUCTIONS FOR PATIENT FOLLOW-UP You will keep an individual log of the FNA procedures you perform during your Cytology Fellowship year. You will also be expected to contact each patient by telephone within 5 working days of the FNA procedure to ask the patient about his/her post-procedure symptoms and their management/resolution, and to obtain feedback about their overall experience with the FNA procedure.
Sample dialogue with patient at the end of FNA appointment:
CytoFellow: Would you mind if I called you in a few days to check in and see how you are doing?
Patient: Okay.
CytoFellow: What would be a good phone number for me to reach you at?
Patient: 617-555-1212.
CytoFellow: Thank you.
Sample follow-up telephone dialogue with patient after FNA procedure:
Cyto Fellow: Hi Mr. Smith. This is Dr. Kerr from MGH. I performed the FNA biopsy procedure on your [body site] last Wednesday. I am calling to see how you are doing. Did you have any discomfort or pain at the biopsy site? How was your overall experience with our FNA team/the biopsy procedure? Etc.
Document the following information using an Excel file spreadsheet using the corresponding column headings (underlined):
1. Patient MRN
2. Date of FNA procedure
3. Body site sampled
4. Was patient amenable to being called?
5. Date of phone call to patient
If patient was successfully contacted, record the following:
6. Complications
When the patient got home, did s/he experience pain (if so, record severity using scale of 1 to 10), bleeding, or bruising? If so, how did s/he treat the symptom(s)? Did symptoms resolve? (e.g. “I had pain 6 out of 10 that evening, but it went down to a 2 out of 10 after I took some Tylenol, and was gone the next morning.”)
7. General Comments
Patient feedback regarding overall experience (e.g. “I was happy that you could see me so soon.”)
Sample Excel sheet
Patient MRN
Date of FNA
Body site
Ok to call?
Date of Phone Call
Complications (pain, bleeding, bruising)
General Comments
9406822
1/1/2012
Left arm
Yes
1/4/12
Pain overnight (7/10), relieved with ice pack, no pain next day, slight bruising
“Dr. Chebib was really nice.” “I appreciate you checking in.”
Recommended Reading
Cibas, E. S. and B. S. Ducatman (2009). Cytology : diagnostic principles and clinical correlates. Philadelphia, PA, Saunders/Elsevier.
Ali, S. Z. and E. S. Cibas (2010). The Bethesda system for reporting thyroid cytopathology : definitions, criteria, and explanatory notes. New York, Springer.
Solomon, D. and R. Nayar (2004). The Bethesda System for Reporting Cervical Cytology: Definitions, Criteria, and Explanatory Notes, Springer.
Bibbo, M. and D. Wilbur (2008). Comprehensive Cytopathology: Expert Consult: Online and Print, Elsevier Health Sciences.
Gupta, P. and Z. Baloch (2011). Cytohistology: Essential and Basic Concepts, Cambridge University Press.
DeMay, R. M. (2012). The Art & Science of Cytopathology, ASCP Press.
Cytology service page
Rotation 2 (2 weeks)
Rotation-2 curriculum is advanced, and intended to be completed during two weeks of AP-2.
Warning: Display title "2-1 Cytology laboratory management" overrides earlier display title "1-17 Palpable Fine Needle Aspiration Biopsy: Procedure, Techniques, and Specimen Handling<b></b>".
- Lab Management: Organizations Lecture (Wait until the very end of the lecture to take a short quiz.)
- Lab Management: Specimen Preparation Lecture (Wait until the very end of the lecture to take a short quiz.)
Regulatory agencies and guidelines
- MGH
- CLIA88
- Joint Commission (JC)
- College of American Pathologists (CAP)
- Department of Public Health (DPH)
Laboratory Inspections Critical values in cytology: MGH Policy Quality management plan
a. Quality control/quality assurance
- 10% rescreen
- Retrospective rescreen
- Cytologic-histologic correlation
- Annual statistics
- Workload records
b. Competency assessment
c. Proficiency testing
d. Continuing Education
e. Performance evaluation
Policy and procedure manuals
Billing Cytology Laboratory Budget Operations
- Salary
- Employee compensation
- Employee benefits
- Expenses
- Lab
- Office
- Service Contracts
- Rentals or Leases
- Regulatory fees and permits
- Maintenance
- Telephone
- Laundry
- Freight
- Revenue
- Cost per test
- Volume
- Inpatient
- Outpatient
Capital
- Purchase
- Reagent Rental
- Lease
Cytology service page
Warning: Display title "2-2 Gyn Cytology" overrides earlier display title "2-1 Cytology laboratory management".
HPV Testing Methods; additional tests
- Qiagen Hybrid Capture II : RNA:DNA Hybrid Capture
- Cervista: Invader Technology
- Roche Cobas 4800 Real-Time PCR
- Tigris Aptima: mRNA hybrid capture
- In situ Hybridization
- Biomarkers: -p16INKA4a/CINtec Dual Stain
Image analysis assisted screening
Guidelines for management of patients with abnormal cervical cytology results
Advanced gyn cytomorphology cases:
- HSIL variants/mimics
- Adenocarcinoma variants/mimics
- Radiation change
Cytology service page
Warning: Display title "2-3 Immunochemical stains in cytopathology" overrides earlier display title "2-2 Gyn Cytology".
Immunochemistry is used frequently in the work up of cytology samples, but given that most immunochemical staining protocols were developed for histologic sections and controls are usually histologic tissue sections, one needs to be aware of the factors that will impact immunocytochemical stains.
For a given cytologic preparation (cell block, direct smears, liquid based cytology) the issues with immunochemical stain will vary.
Cell block
Direct smear
Liquid based cytology
Cytology service page
Warning: Display title "2-4 Fluids: Ancillary testing" overrides earlier display title "2-3 Immunochemical stains in cytopathology".
Immunocytochemistry panels
Molecular pathology
Cytology service page
Warning: Display title "2-5 Urine: Ancillary testing" overrides earlier display title "2-4 Fluids: Ancillary testing".
Point of care tests
Immunocytochemistry panels
FISH
Cytology service page
Warning: Display title "2-6 Pancreatic cytology" overrides earlier display title "2-5 Urine: Ancillary testing".
Cyst analysis
Ancillary testing
Immunocytochemistry panels
Molecular pathology
Cytology service page
Warning: Display title "2-7 Respiratory cytology: Ancillary testing" overrides earlier display title "2-6 Pancreatic cytology".
Microbiology cultures
Immunocytochemistry panels
Molecular pathology
Cytology service page
Warning: Display title "2-8 Thyroid cytology: Molecular testing" overrides earlier display title "2-7 Respiratory cytology: Ancillary testing".
Cytology service page
Warning: Display title "2-9 Lymphoid lesions" overrides earlier display title "2-8 Thyroid cytology: Molecular testing".
Advanced cytomorphology
Flow cytometry
Molecular testing
Cytology service page
Warning: Display title "2-10 Thymus and mediastinum" overrides earlier display title "2-9 Lymphoid lesions".
Cytology service page
Welcome to Cytology
Cytopathology is considered a subspecialty of anatomic pathology but in reality the discipline touches on all areas of anatomic pathology, albeit from the vantage point of the individual cell rather than tissue. The cytopathology laboratory functions separately but in parallel with the other anatomic pathology and clinical pathology labs. Correlation of cytopathology findings with test results obtained on concurrent tissue samples and other ancillary tests (biochemical and molecular) helps to improve diagnostic accuracy. Correlation with clinical and radiologic findings is also important. Residency training in cytopathology trains residents to be competent using a stepwise process for each organ system:
- Indications for cytological examination
- How to procure the specimen
- Specimen processing
- Test platforms used
- Reporting terminology
- Cytomorphology of normal cells and pathologically altered cells
The learning goals are in tune with the pathology milestones. The resident is expected to be at level 1 at the start of residency. Therefore the learning objectives are divided into the levels 2, 3 and 4 to correlate with the milestones. Residents may advance at a more or less accelerated pace through these levels.