|
|
| (One intermediate revision by the same user not shown) |
(No difference)
|
Latest revision as of 05:20, July 14, 2020
General
|
Definition: Carcinomas with endocrine differentiation form a family of ductal carcinomas united by the presence of morphological and immunohistochemical findings attributable to (neuro)endocrine differentiation. The family includes: papillary carcinoma with endocrine features (solid papillary carcinoma), cellular mucinous carcinoma, and neuroendocrine carcinoma. Neuroendocrine carcinomas take the form of small cell carcinoma and invasive ductal carcinoma with endocrine features.
Clinical Significance: Carcinomas with endocrine differentiation often afflict older women. Certain types may pursue an indolent course.
Gross Findings: These carcinomas often grow as red-tan masses and demonstrate a soft, delicate consistency. Papillary and mucinous varieties look like conventional papillary and mucinous carcinomas.
Microscopic Findings: The microscopic features vary depending on the nature of the carcinoma, but all demonstrate nuclear characteristics typically seen in endocrine tumors.
Differential Diagnosis: Carcinomas without endocrine differentiation
Discussion: Papillary and mucinous carcinomas showing endocrine differentiation occur commonly. Typical cases are strongly positive for estrogen receptor. Small cell carcinoma of the breast is rare.
|
 Several different types of breast carcinoma showing endocrine differentiation are classified as such. Several different types of breast carcinoma showing endocrine differentiation are classified as such. |
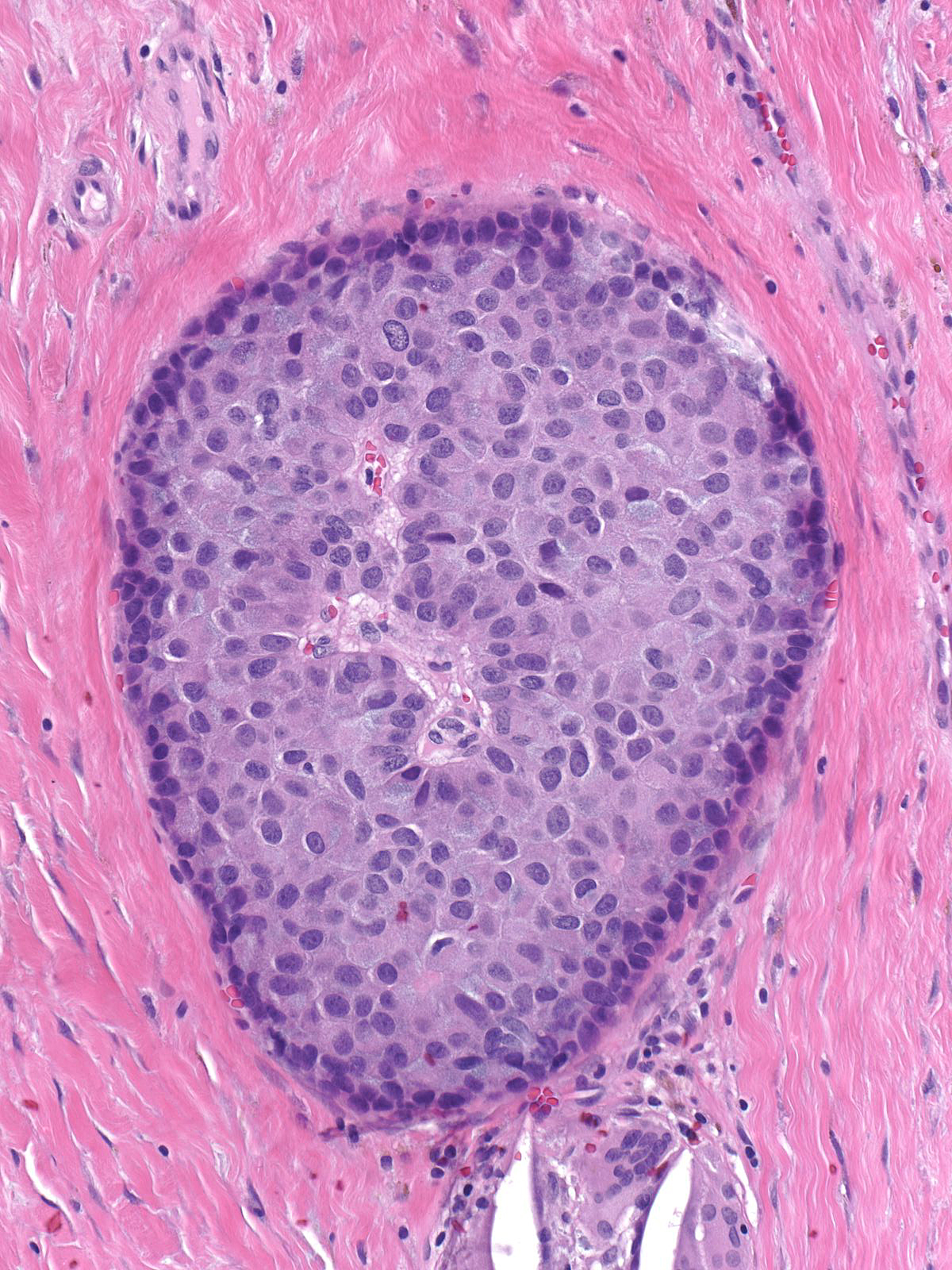
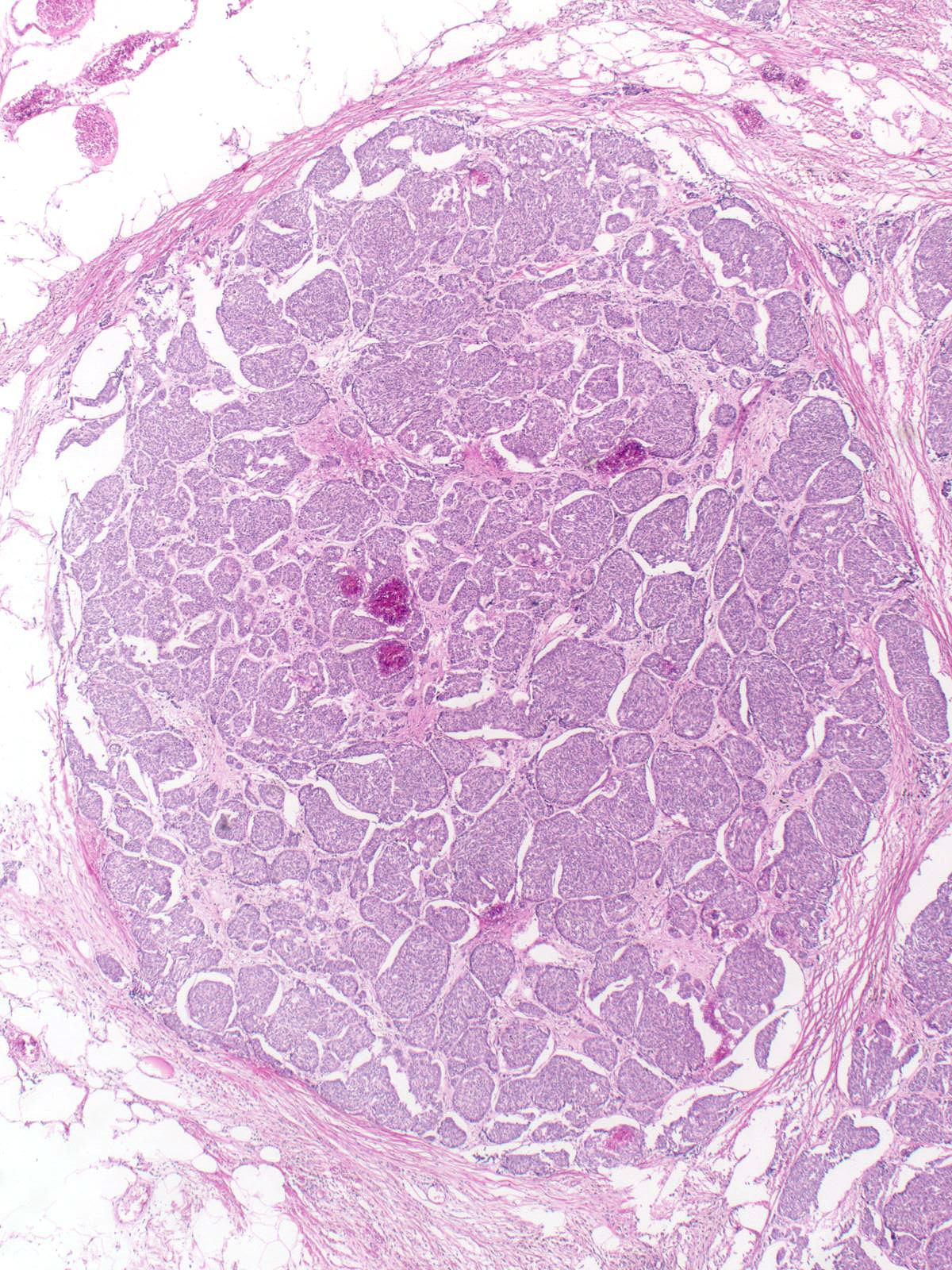
Solid Papillary Carcinoma
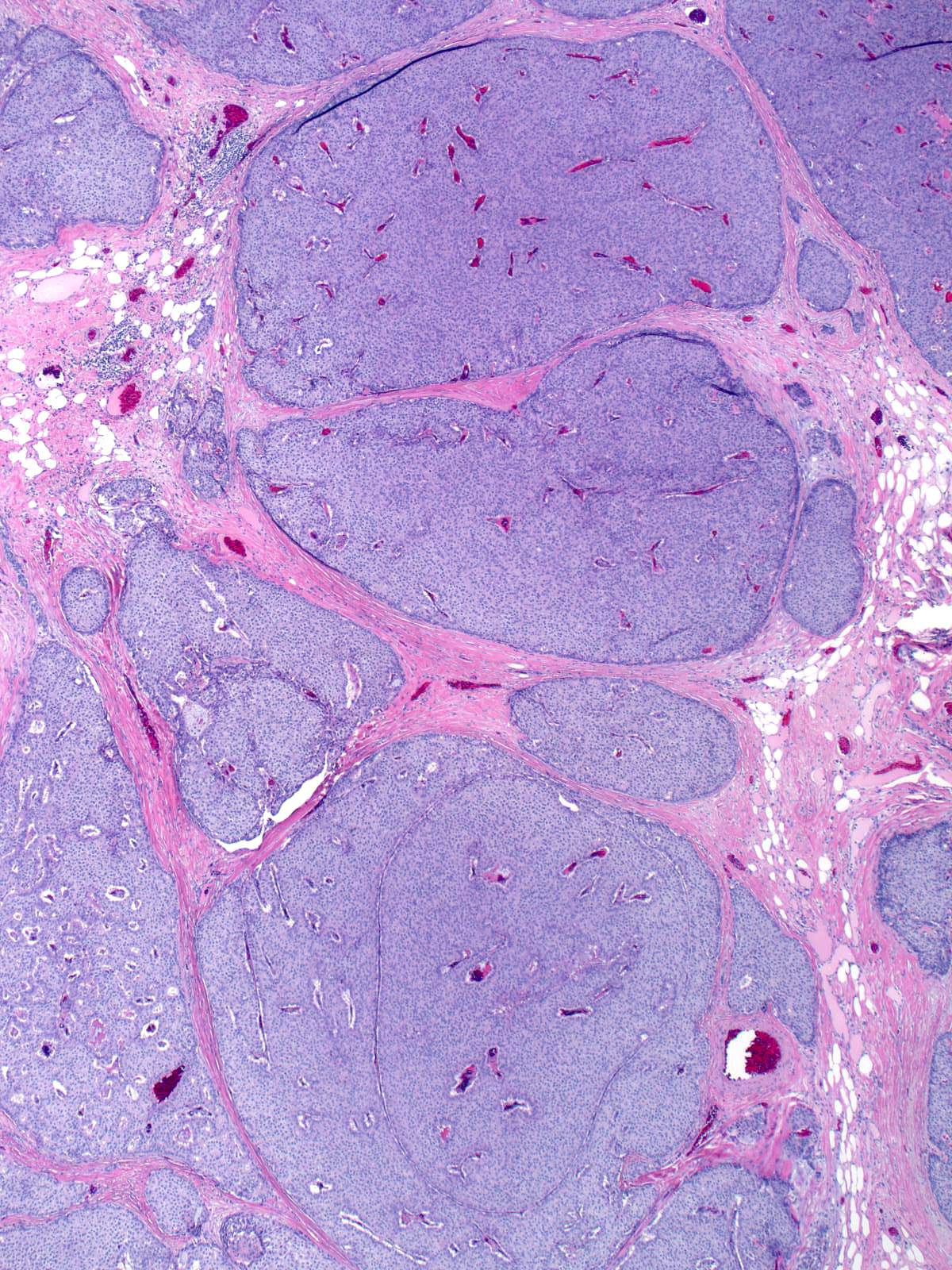
Papillary carcinomas with endocrine differentiation usually display a solid manner of growth.
| The dense packing of the cells may make it difficult to appreciate the tumor's papillary architecture. Capillaries traverse the cellular masses. |
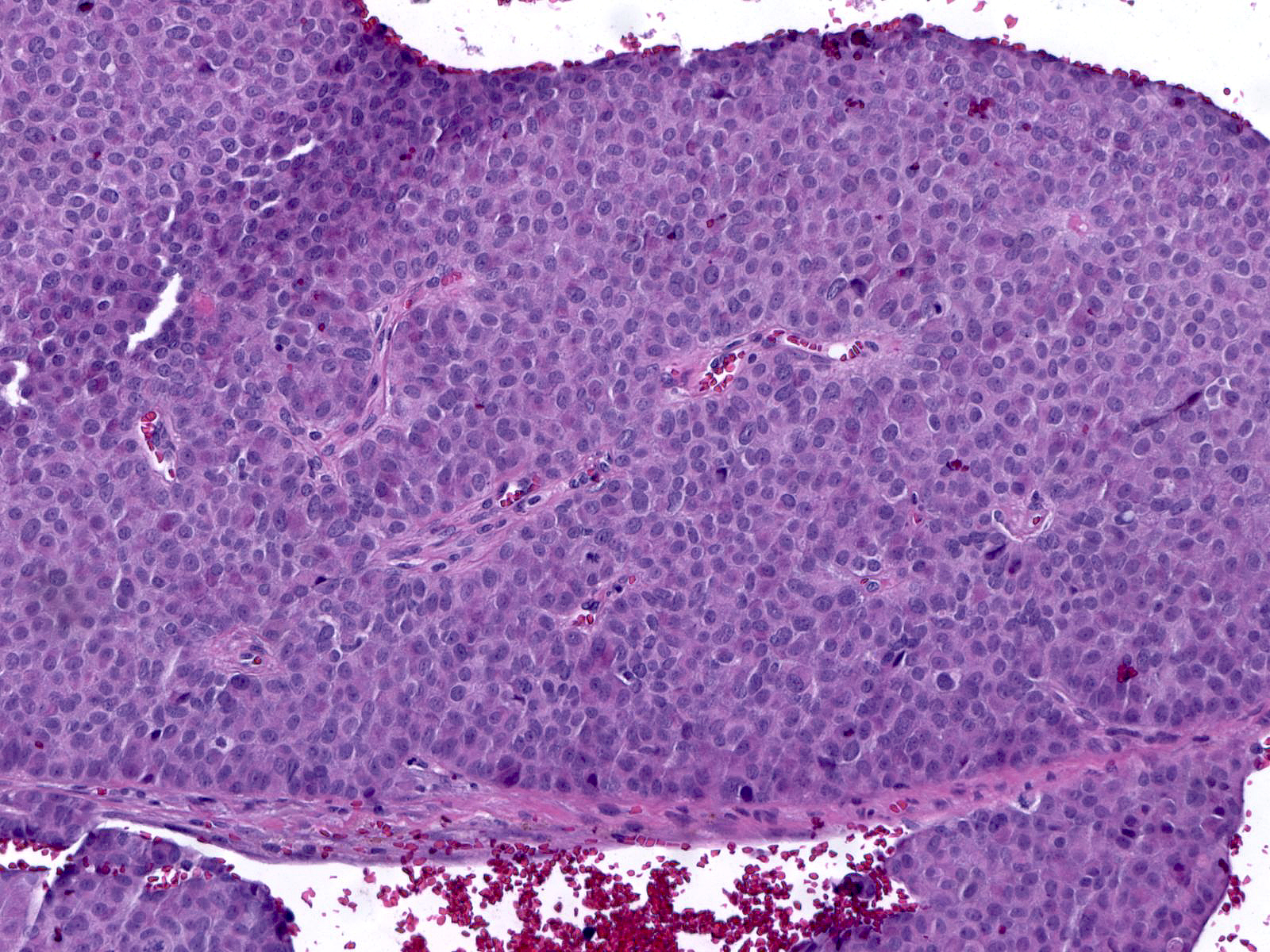 |
|
|
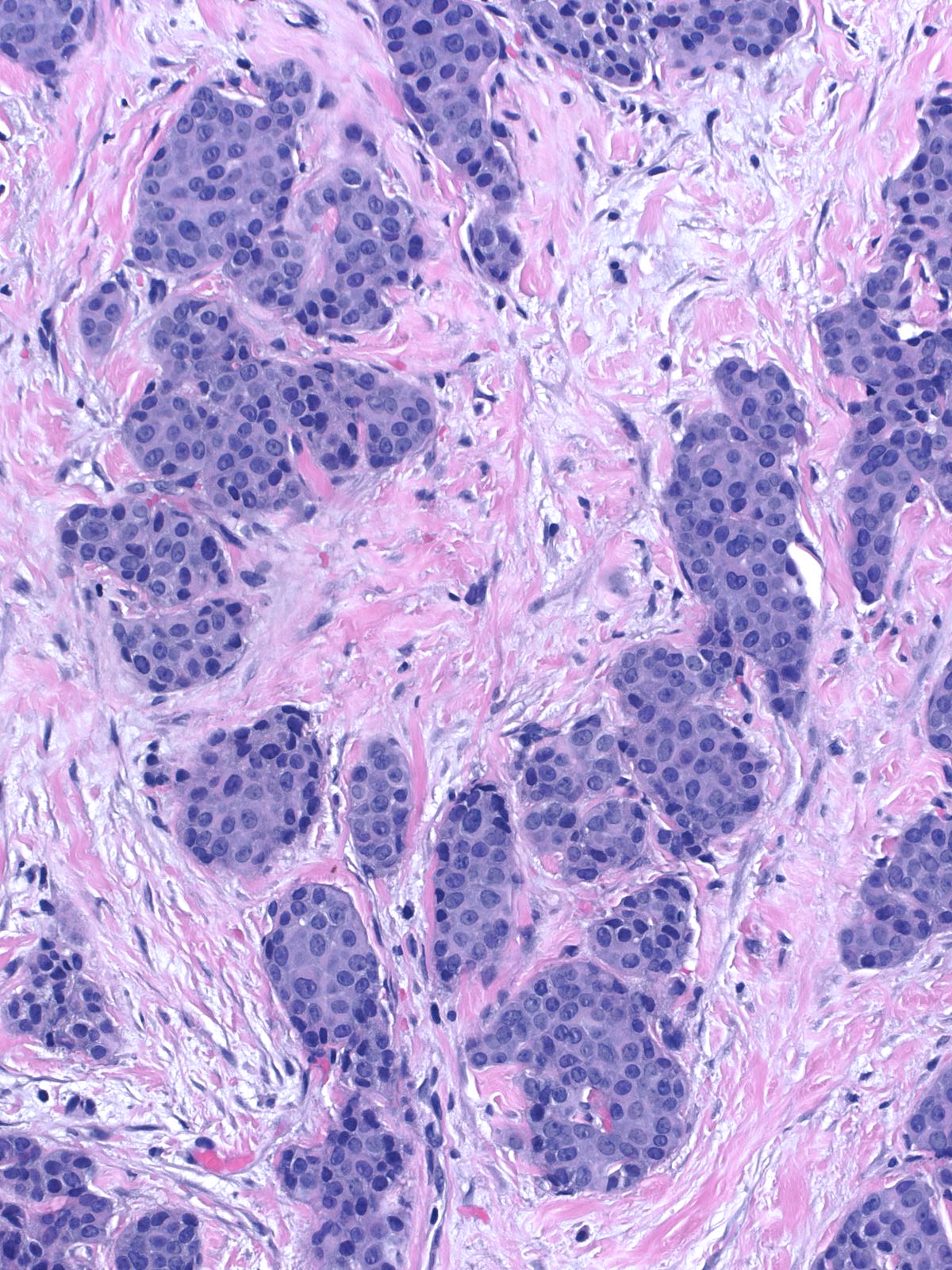
| The mass often consists of clustered rounded or polygonal aggregates of malignant cells supported by a variably robust fibrovascular skeleton. |
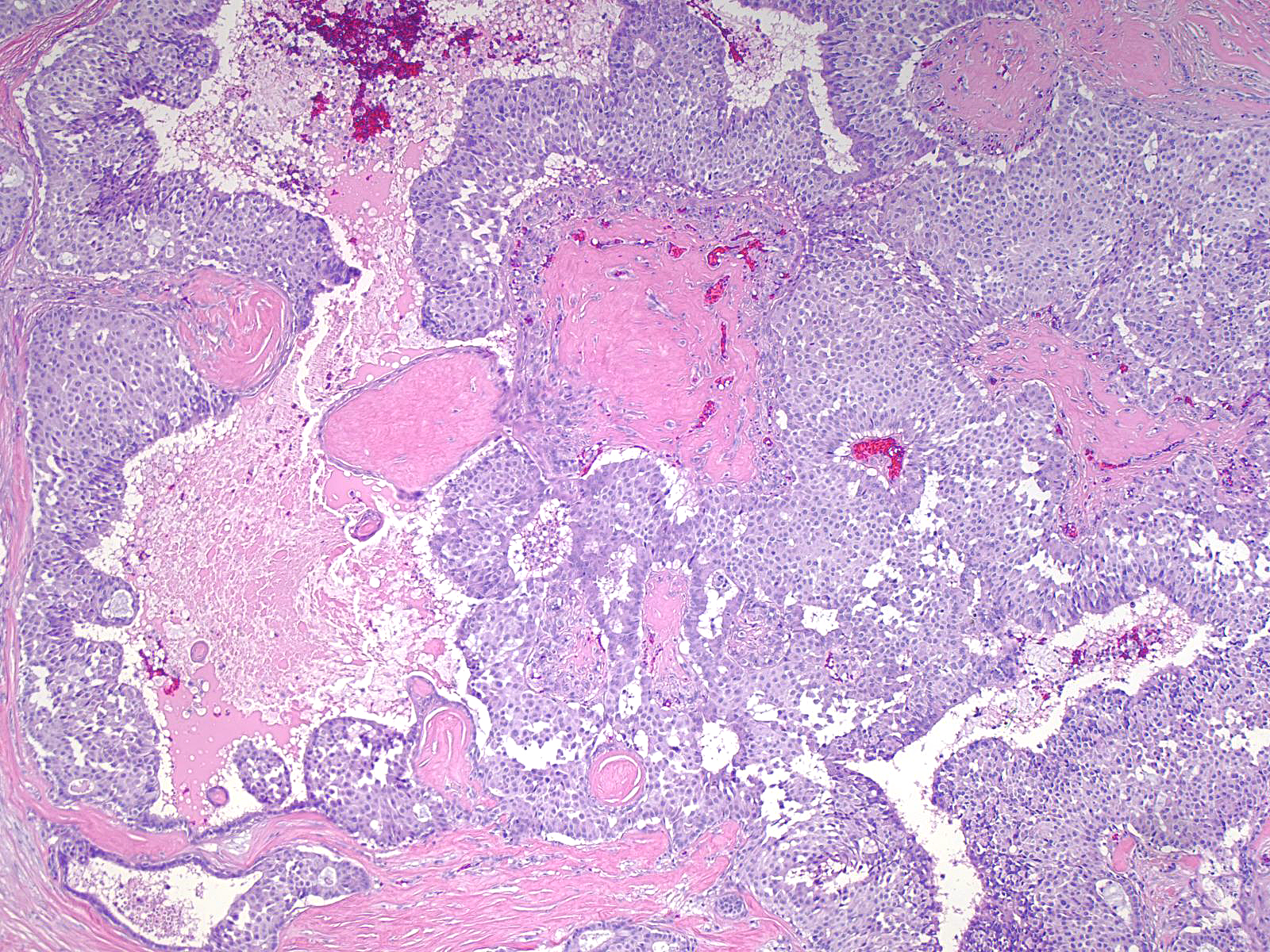 |
|
|
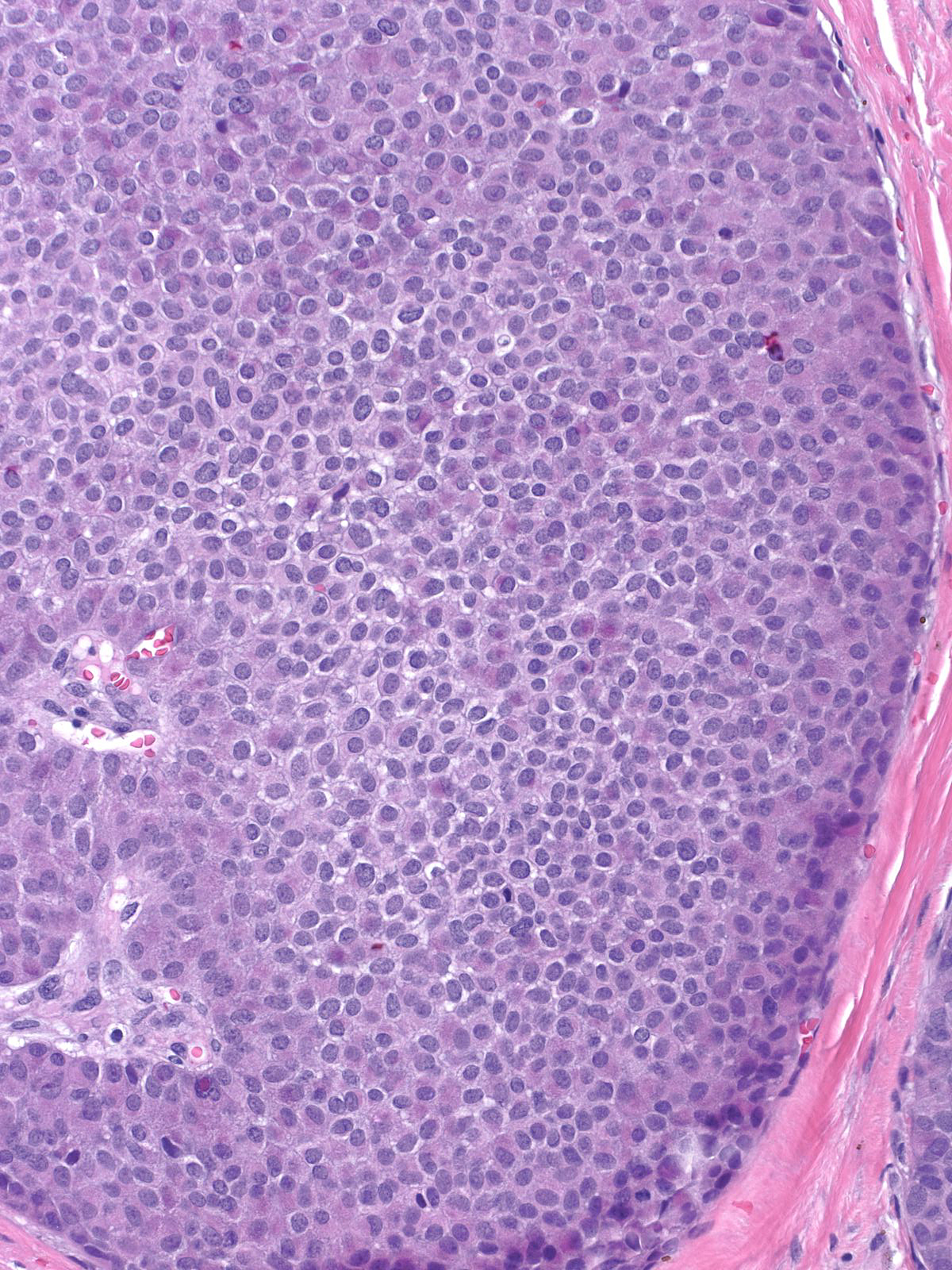
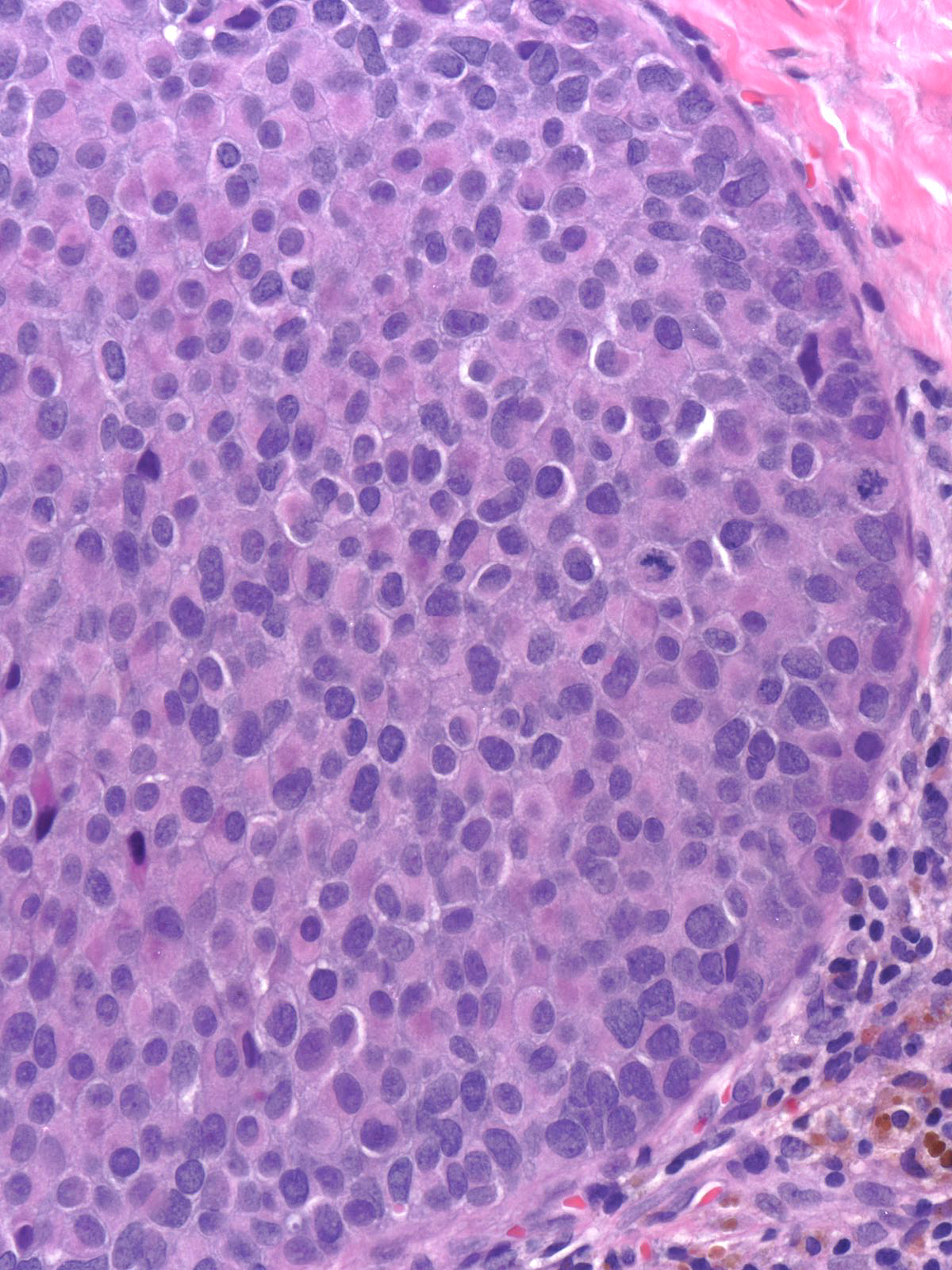
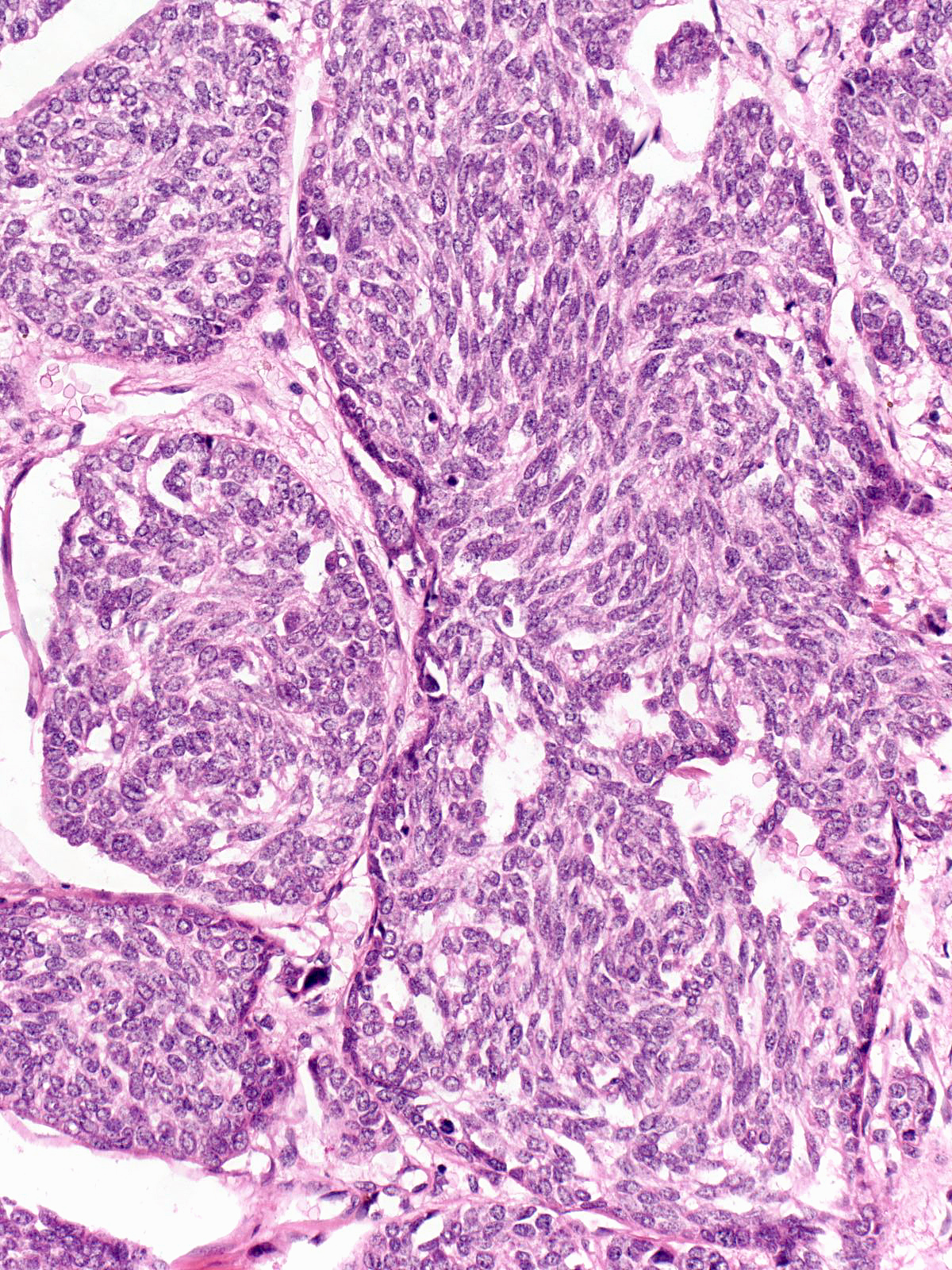
| The carcinoma cells appear bland. Most have rounded shapes, but elongated cells occur frequently. |
 |
|
|
The carcinoma cells contain round, oval, or spindle-shaped nuclei.
They often demonstrate a somewhat greater degree of pleomorphism than one expects for low-grade nuclei, and one typically finds mitotic figures during a short search.
In certain cases, the nuclei exhibit folds and grooves, and one often observes small nucleoli.
| In rare cases, the nuclei contain intranuclear inclusions. The presence of nuclear folds, small nucleoli, and intranuclear inclusions can make it difficult to distinguish these nuclei from those of hyperplastic ductal cells. Attention to findings such as those illustrated below and the results of immunohistochemical staining for CK5/6 will clarify the diagnosis in cases showing confusing nuclear findings. |
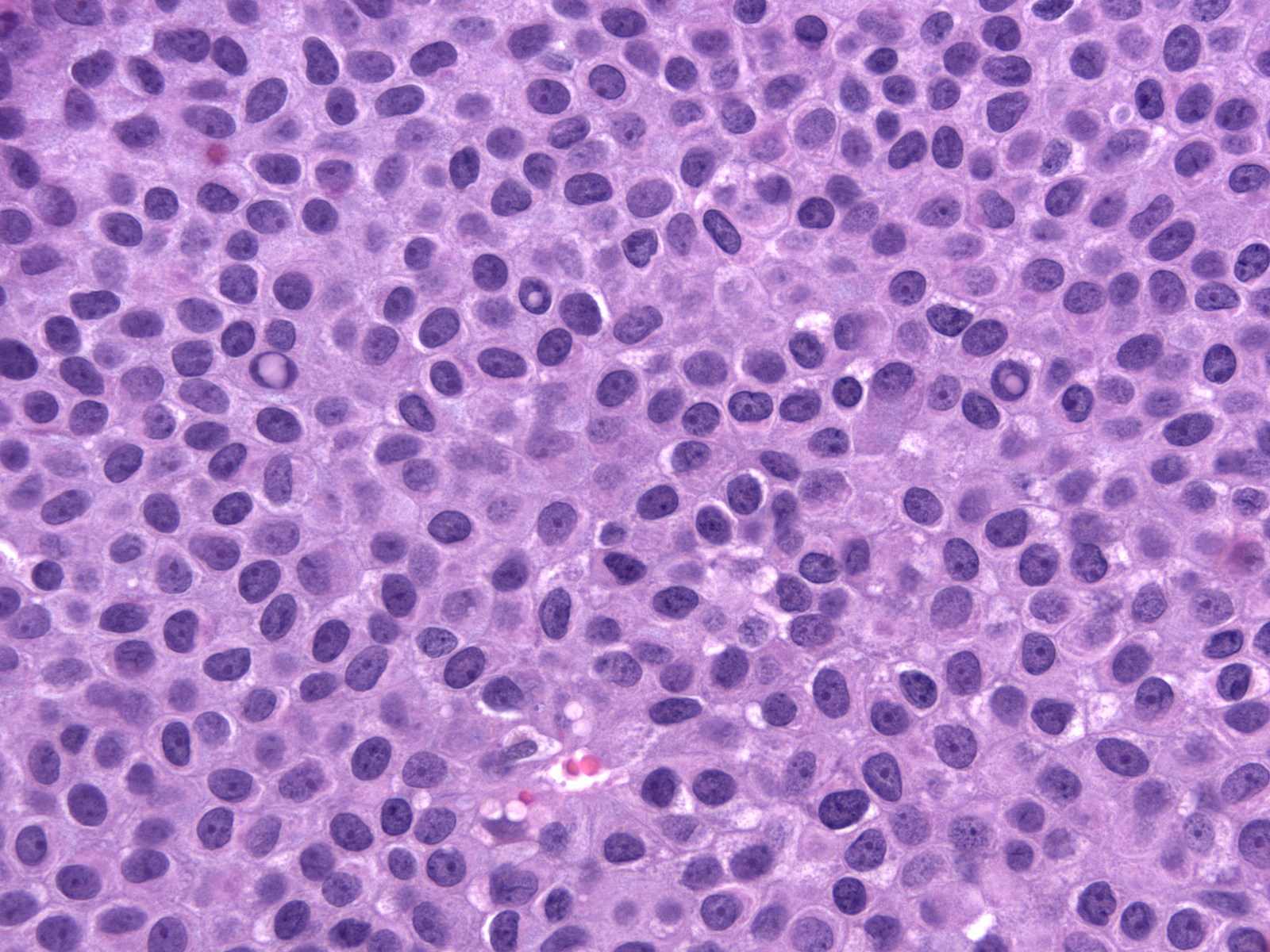 |
|
|
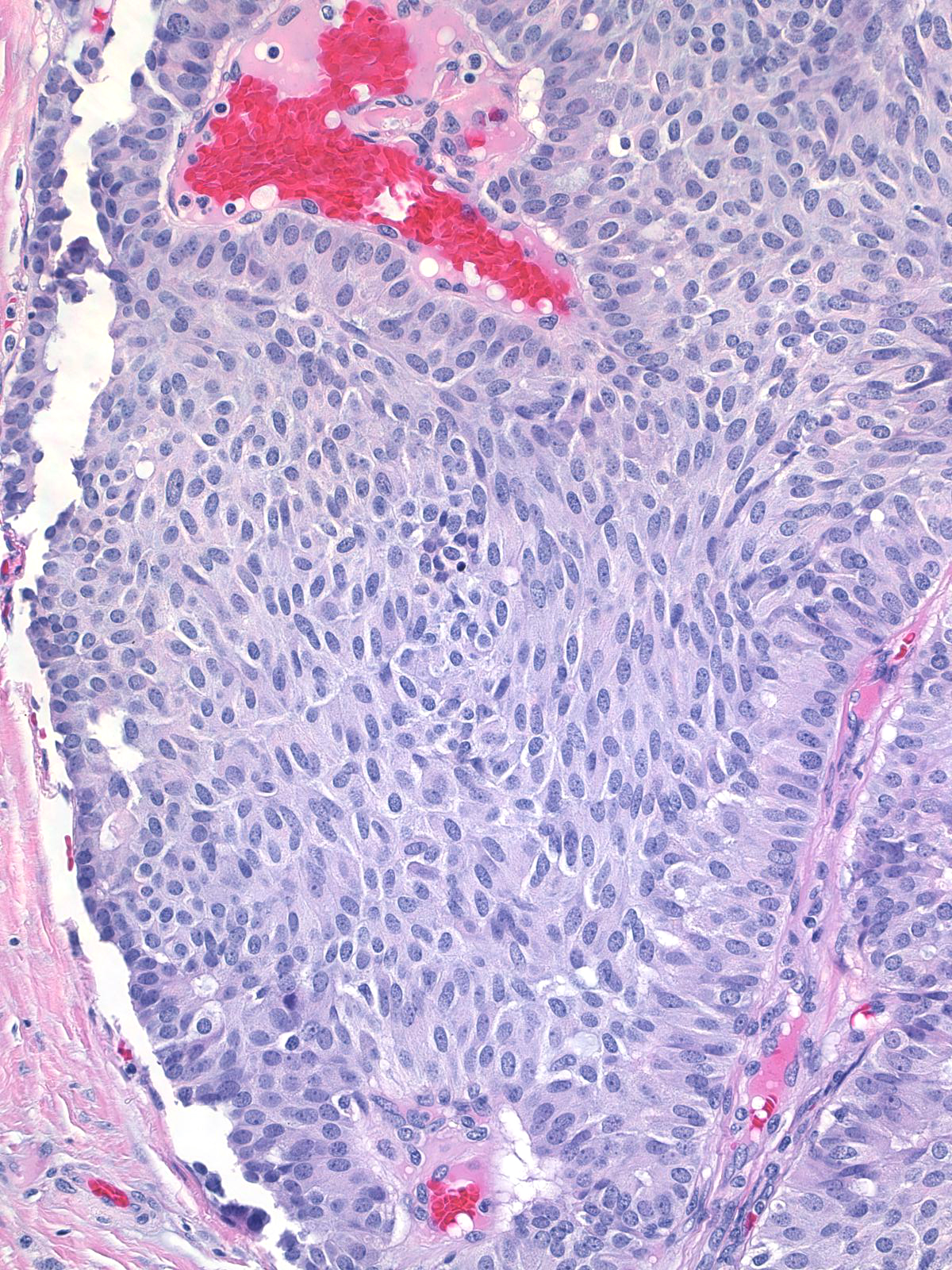
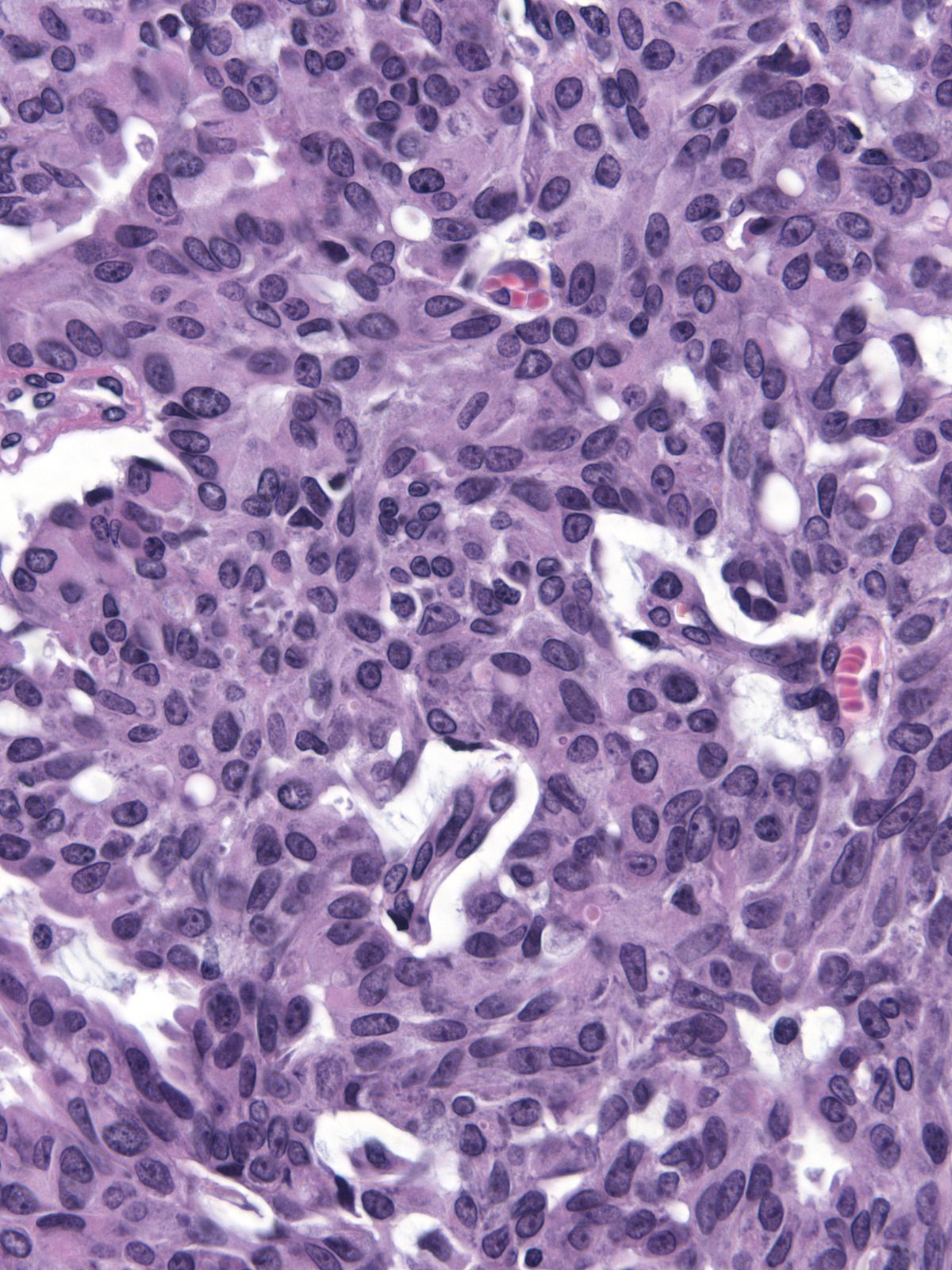
The cytoplasm displays an eosinophilic hue, and one can occasionally see eosinophilic, intracytoplasmic granules.
| The nuclei of the cells along the stromal cores often sit in a parallel array and in a basal position thereby creating a palisade arrangement. (The parallel orientation of the oval nuclei resemble the posts in a palisade). |
 |
|
|
| The cells form cribriform spaces only rarely. |
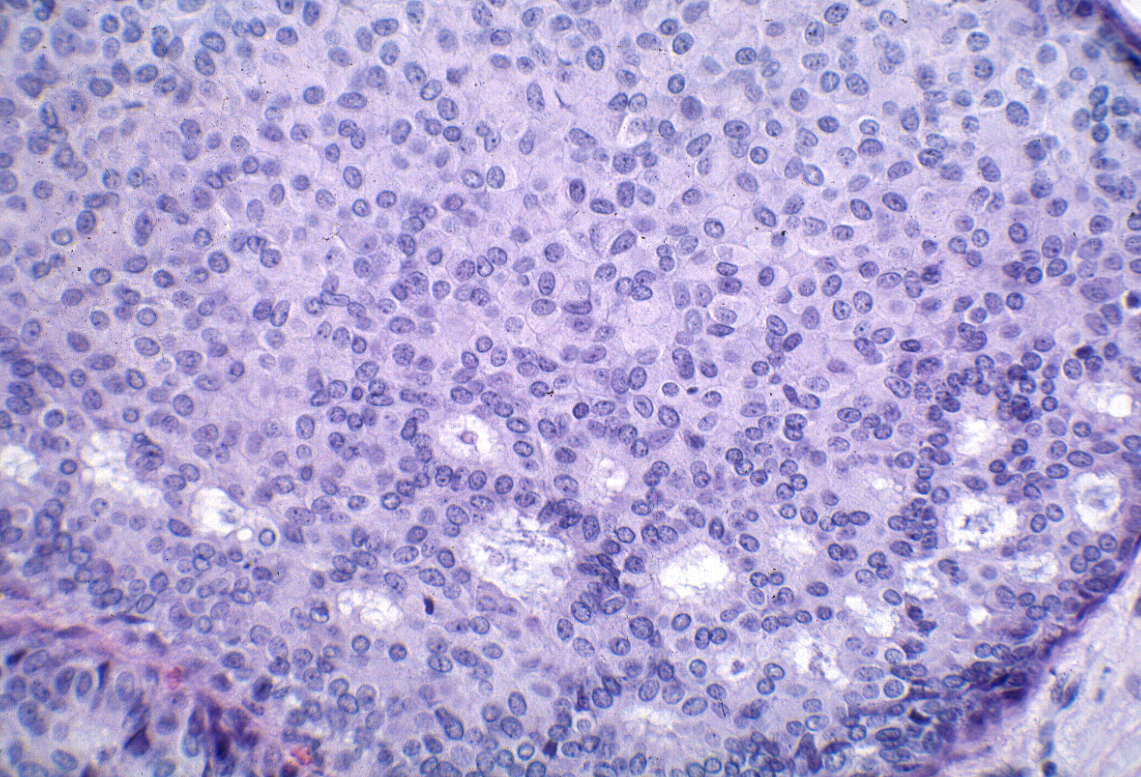 |
|
|
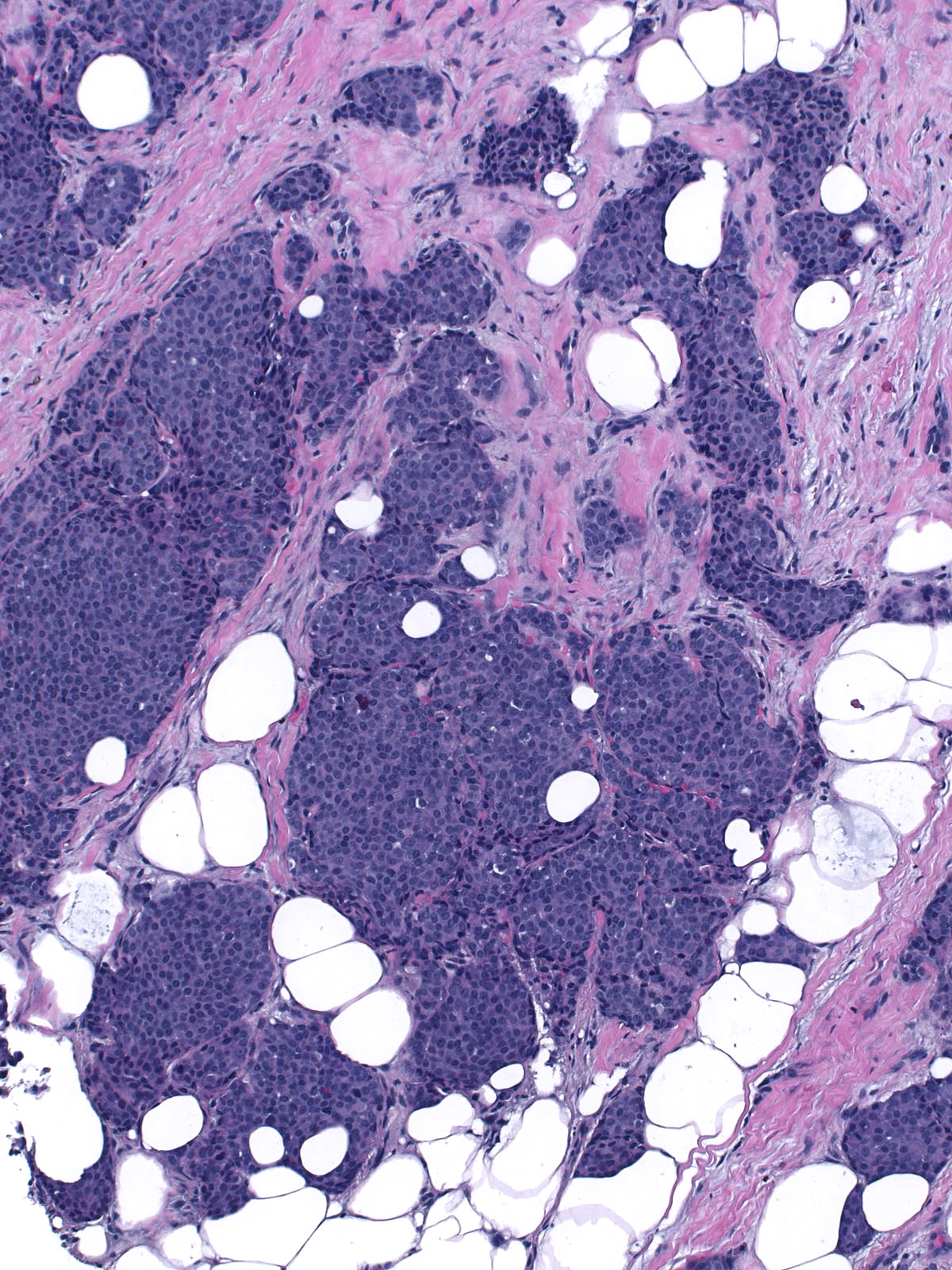
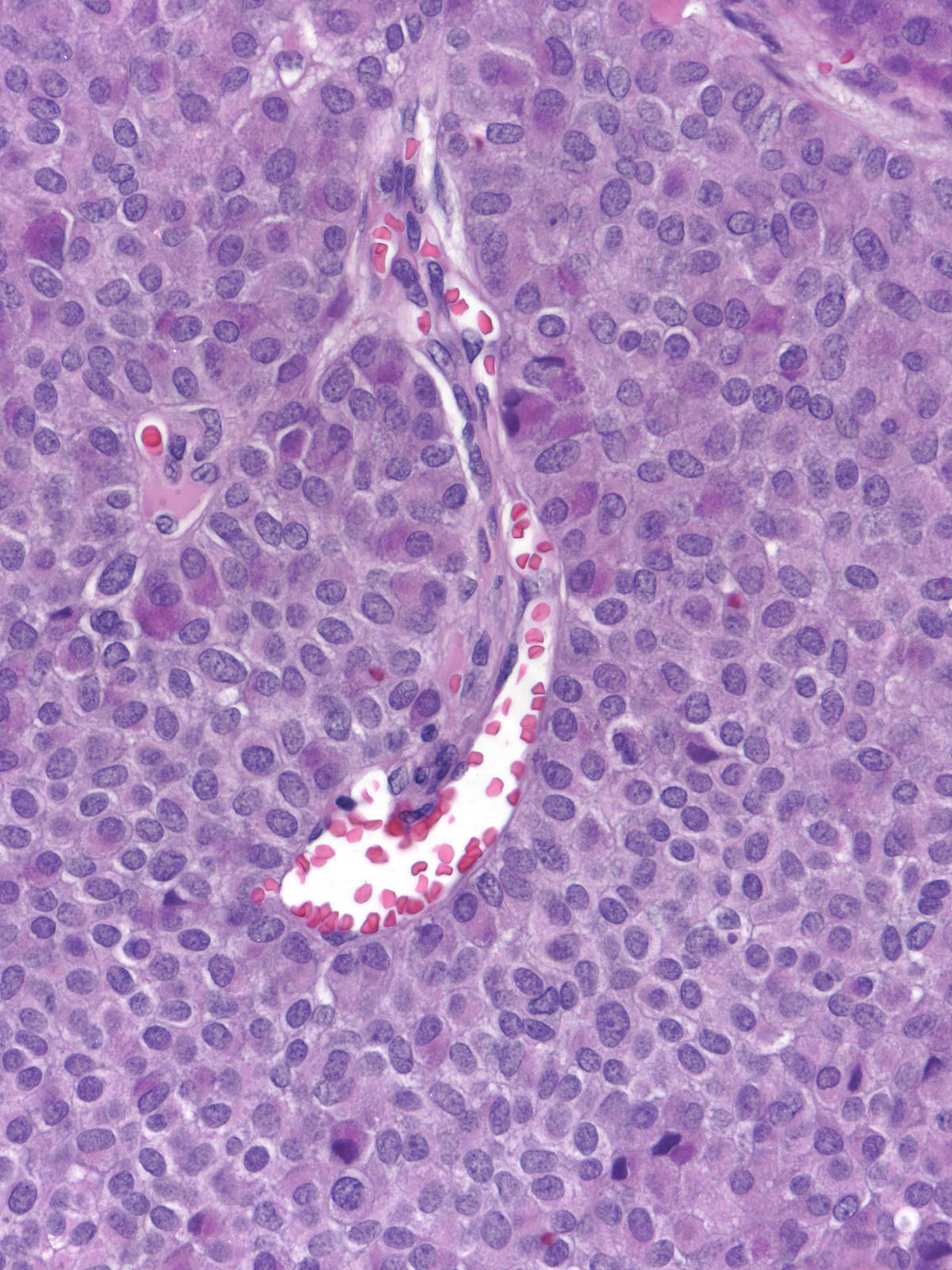
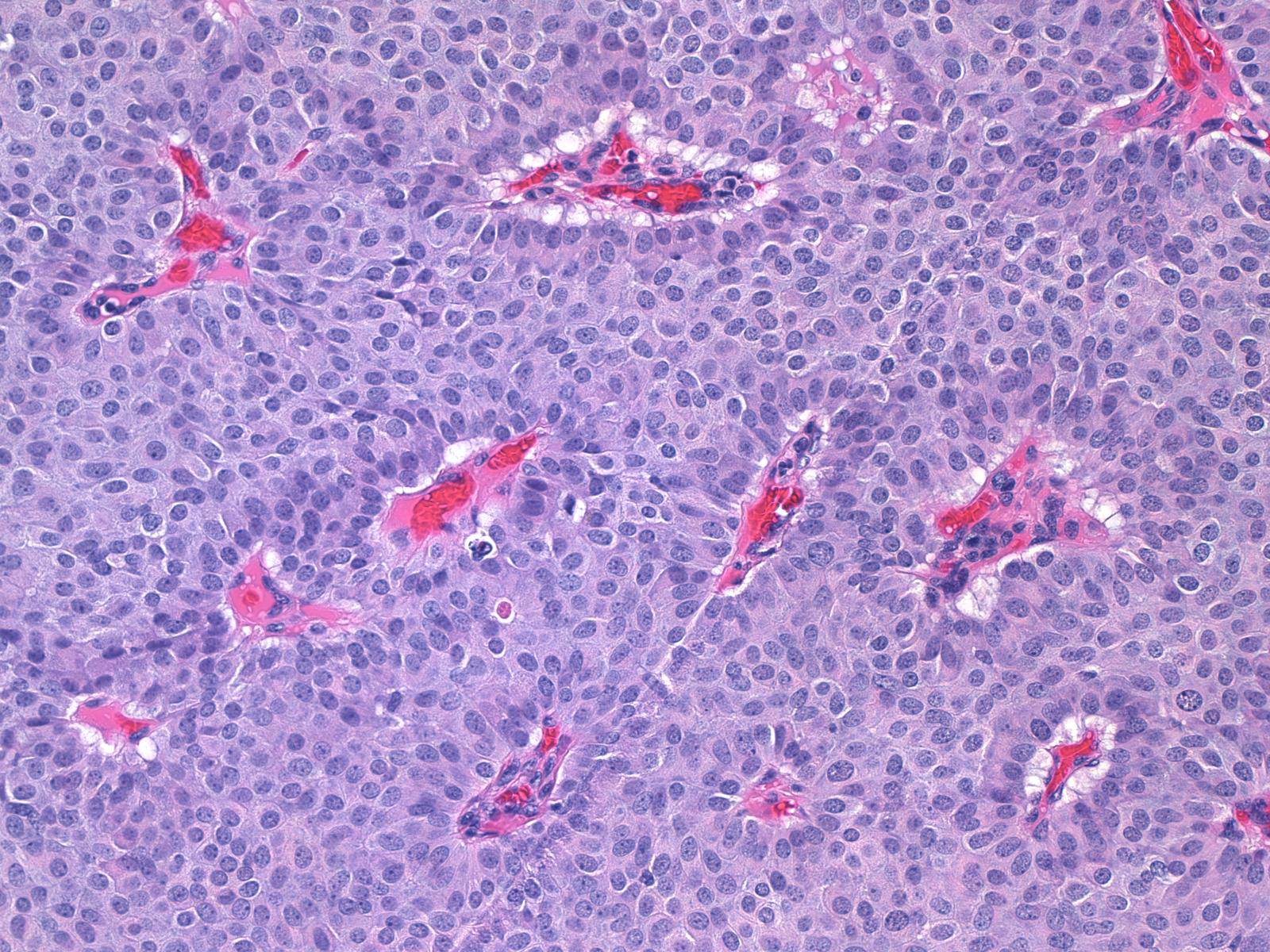
The carcinoma cells typically produce mucin, which usually collects at the interface between the cells and the stroma.
| One often finds blood and hemosiderin in the surrounding stroma. |
 |
|
|
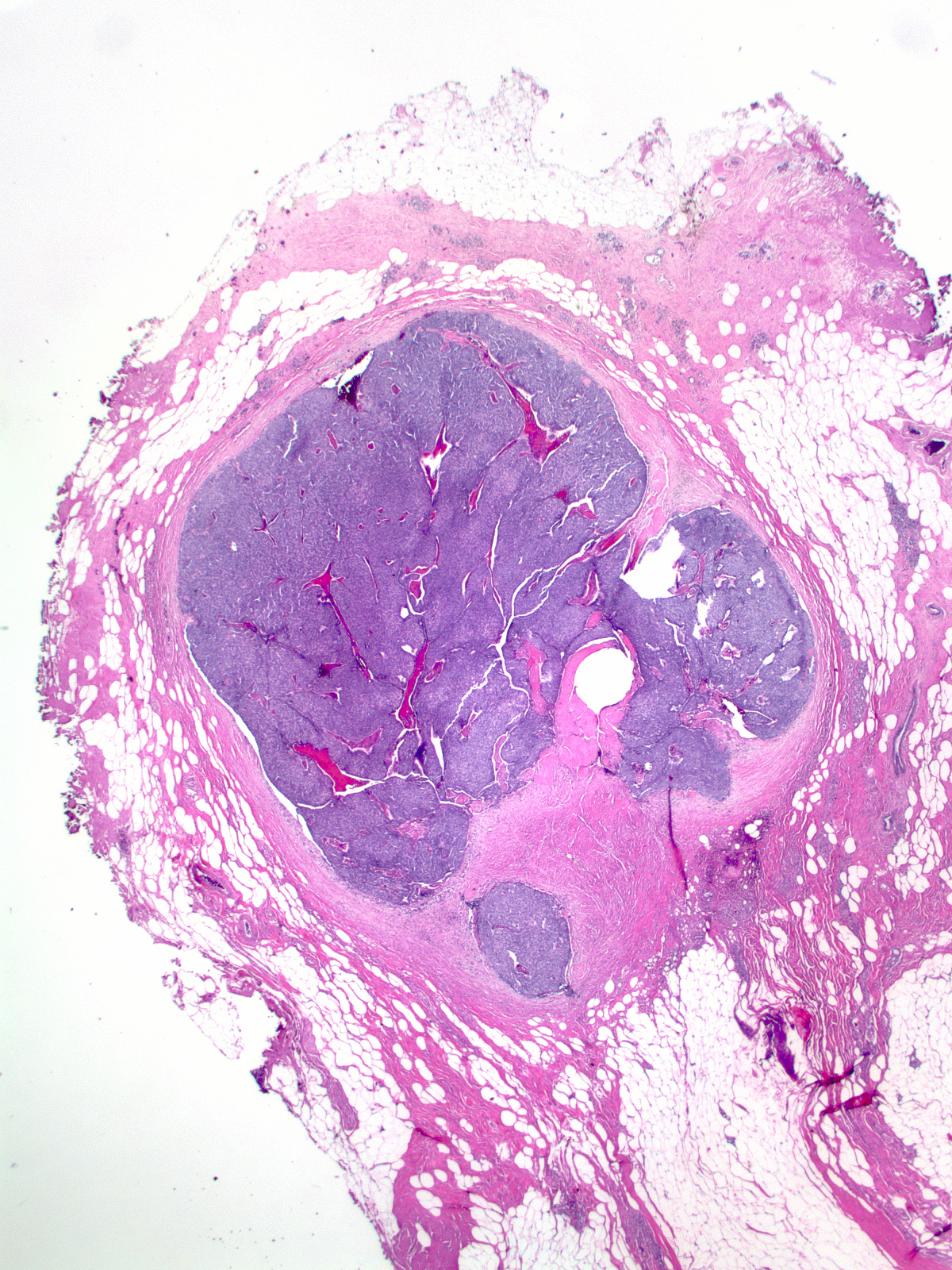
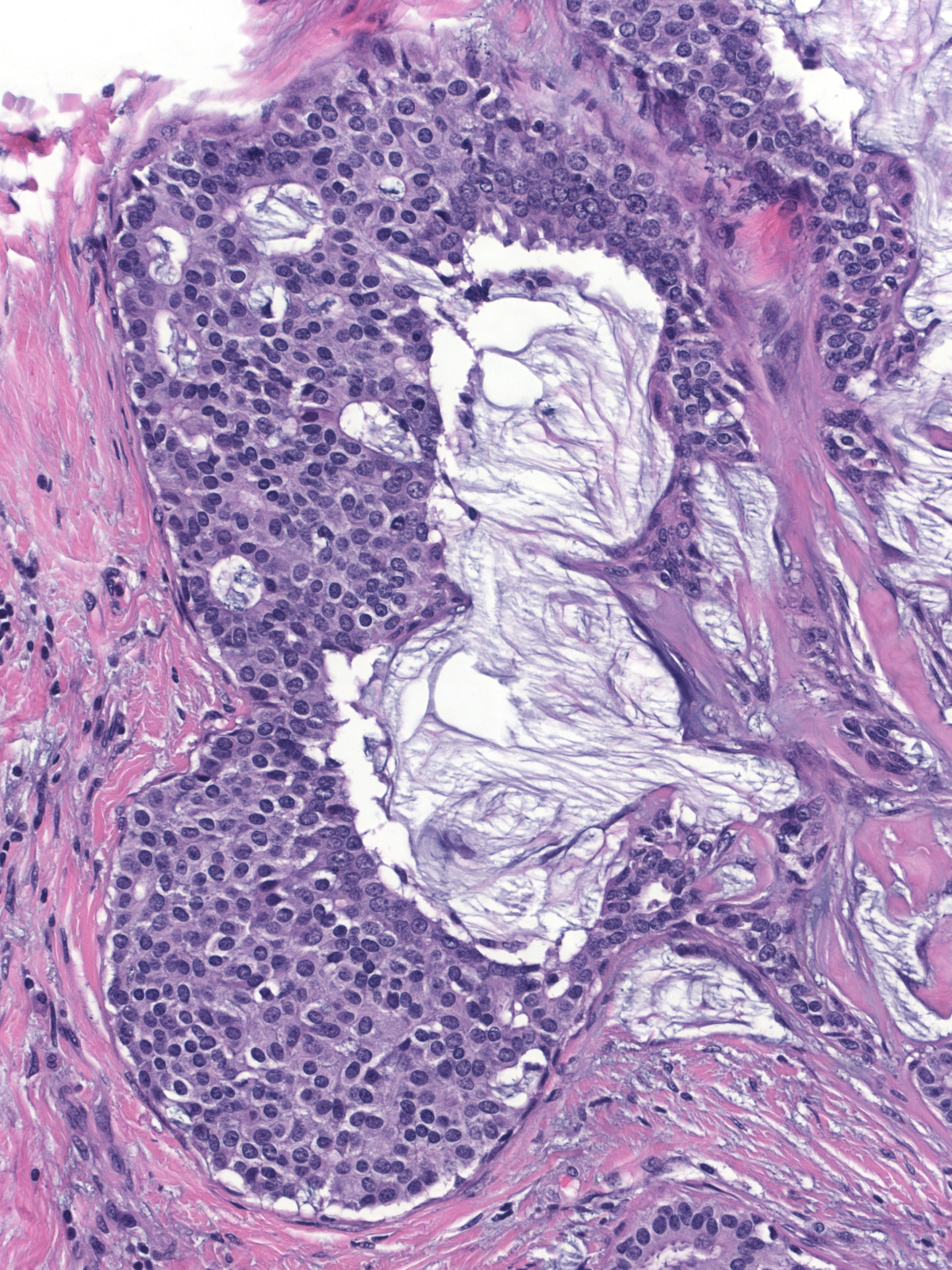
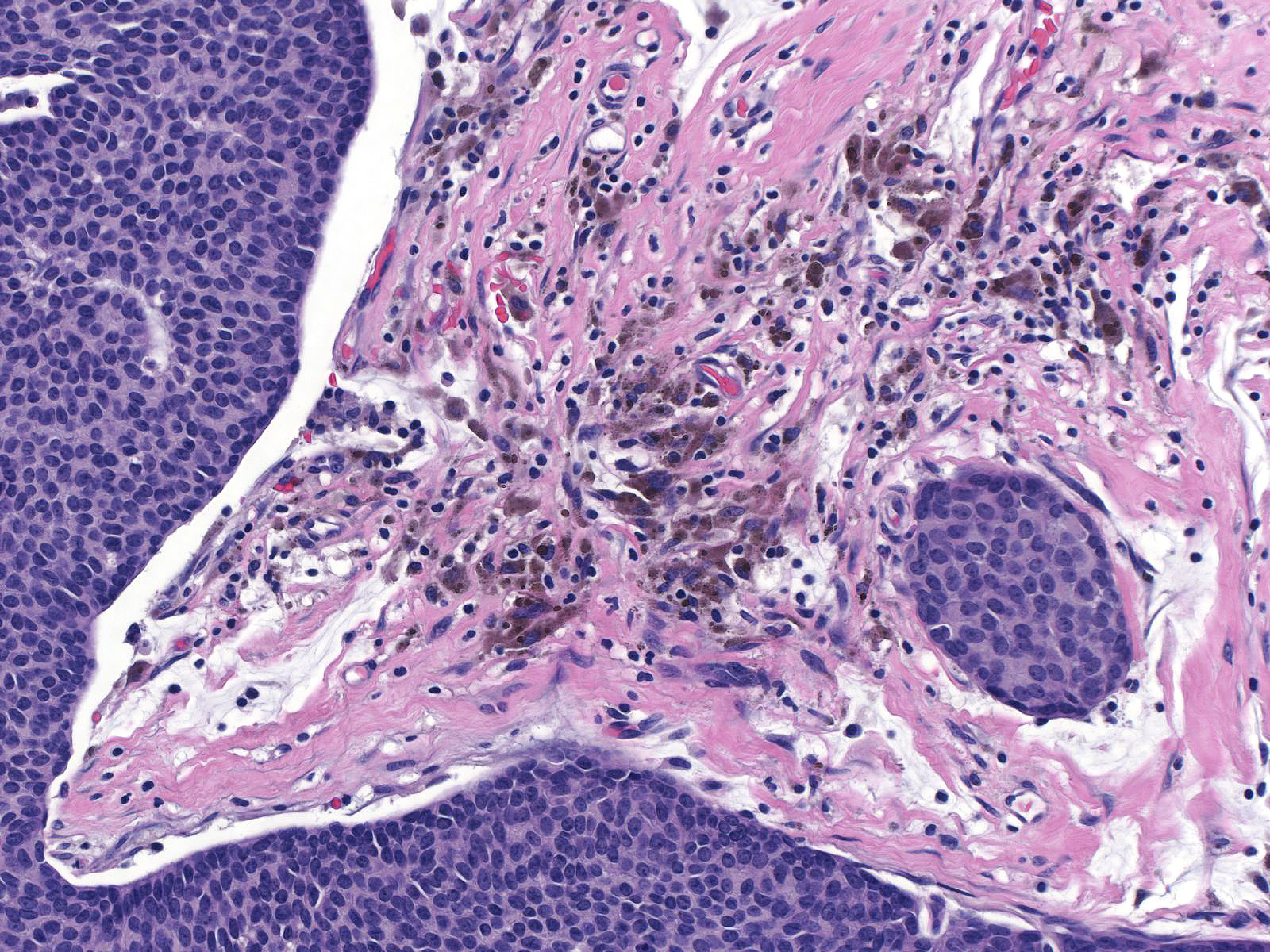
| Papillary carcinoma with endocrine differentiation grows in both noninvasive and invasive forms. The presence of stromal cores in the noninvasive component suggests that the neoplasm might arise in the setting of intraductal papillomas. |
 |
|
|
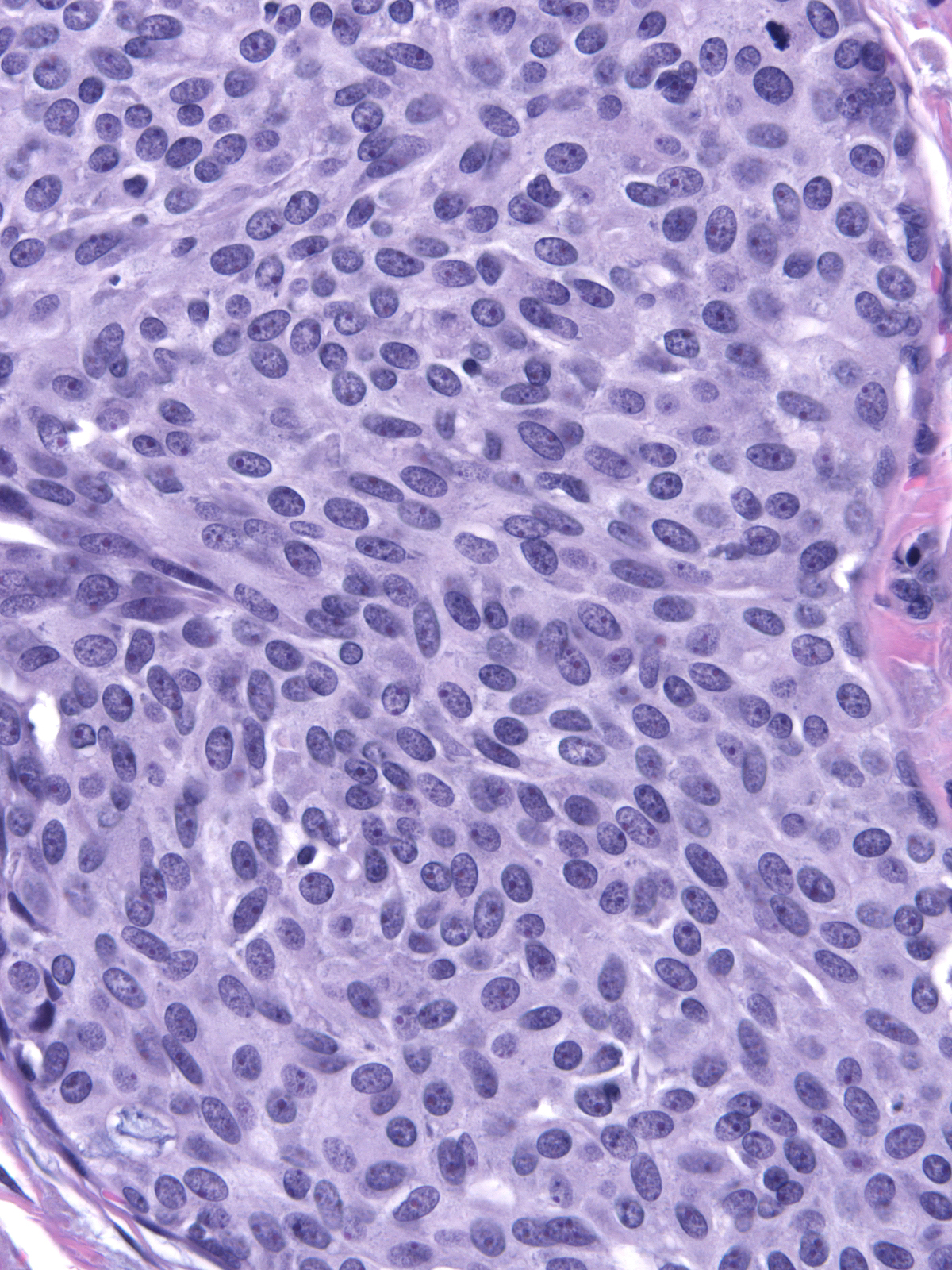
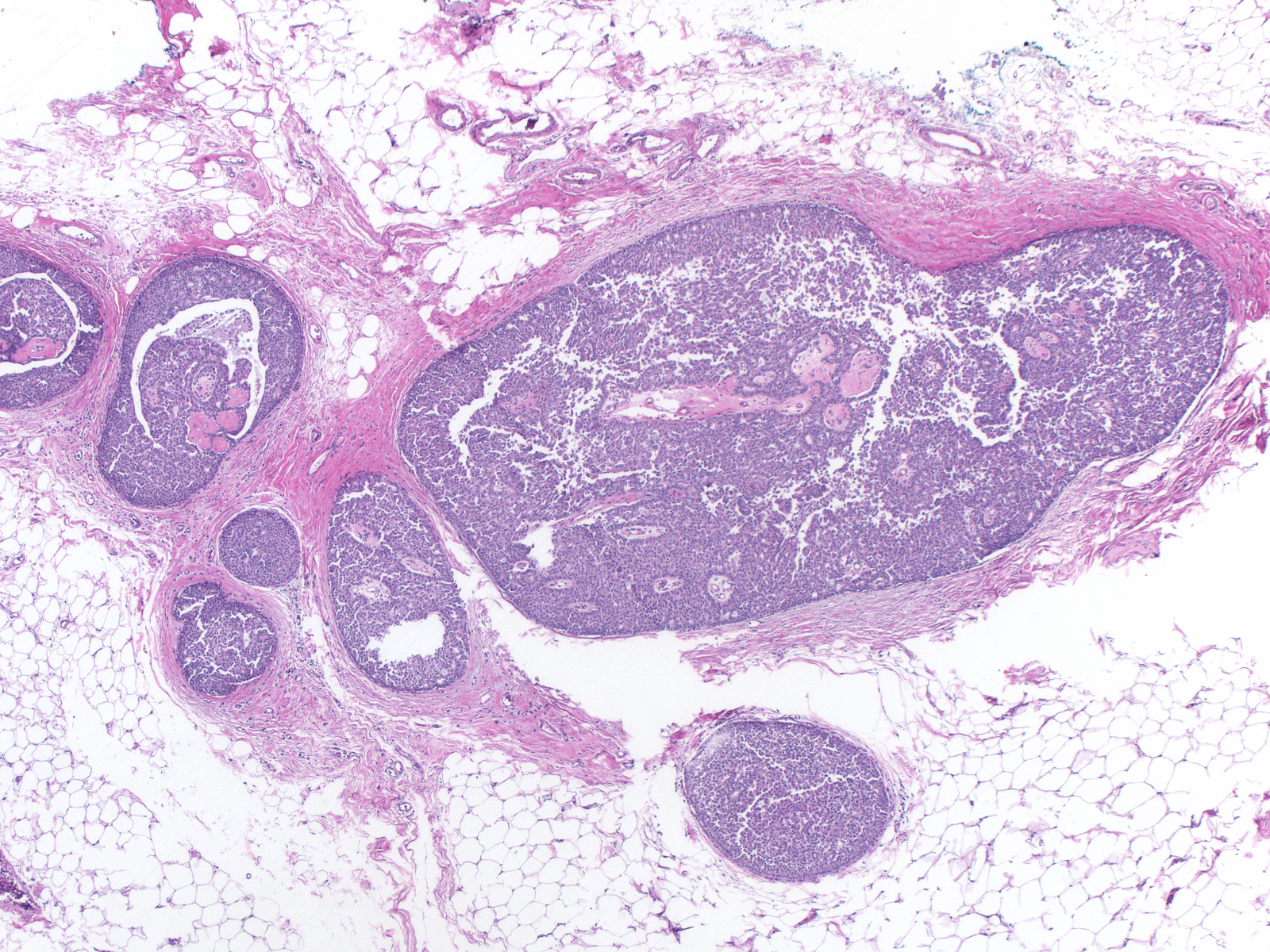
The carcinoma's tendency to grow in large nests makes it difficult to differentiate the noninvasive and invasive forms of solid papillary carcinoma. A lack of an organization consistent with the architecture of the mammary glandular tree, irregularities in the contours of the masses, clusters of cells penetrating the surrounding stroma, and absence of myoepithelial cells, all would support the impression of invasion.
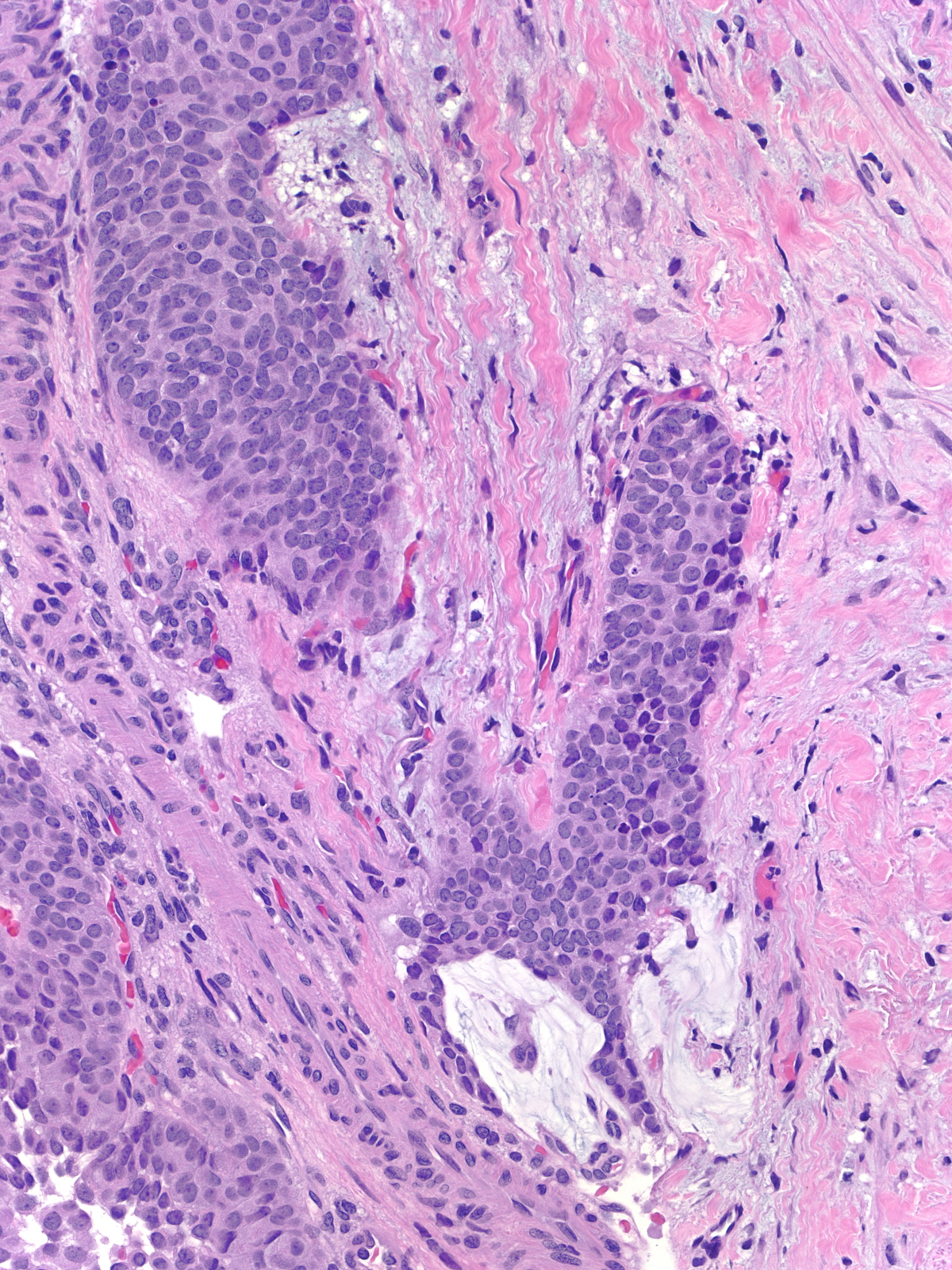 |
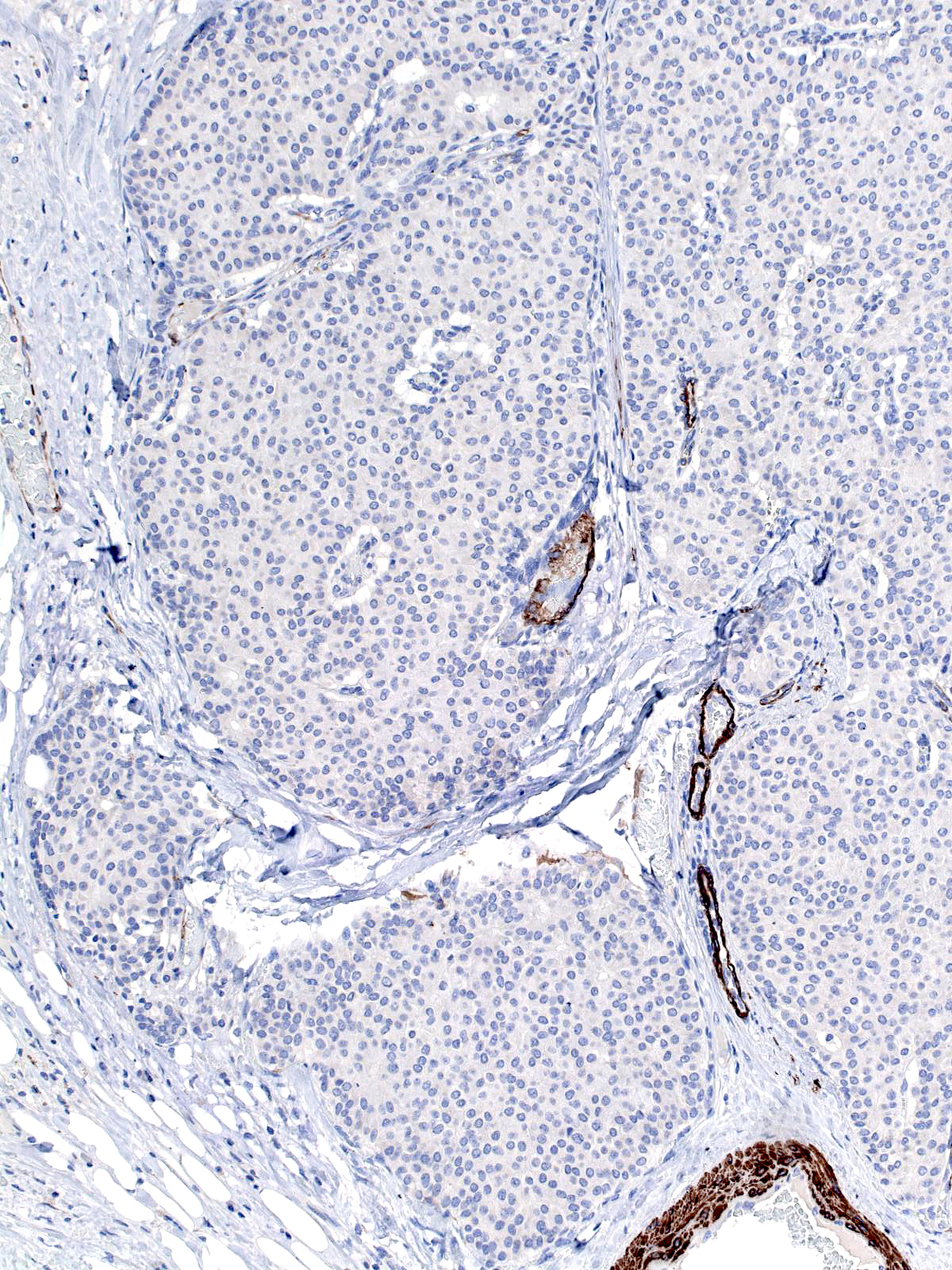 |
| This small invasive focus shows mucin formation. |
A stain for smooth muscle myosin heavy chain does not disclose myoepithelial cells around these solid nests of invasive carcinoma. |
Mucinous Carcinoma with Endocrine Differentiation
| Mucinous carcinomas showing endocrine differentiation usually consists of large aggregates of cells suspended in modest amounts of extracellular mucin. |
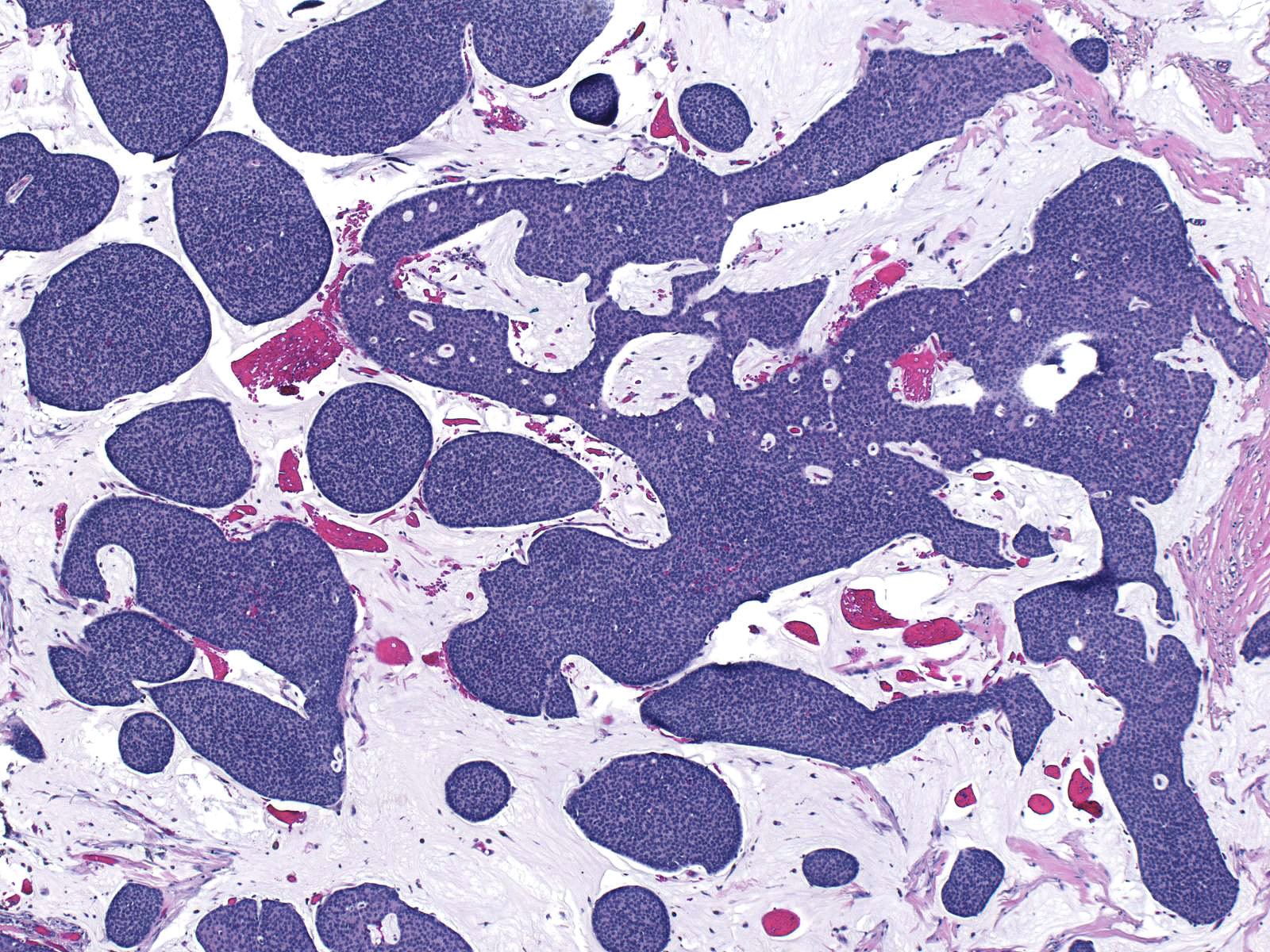 |
|
|
| Mucinous carcinomas with endocrine differentiation are classified as "type B" or as "cellular mucinous carcinomas". Their molecular profile differs from that of type A, or paucicellular, mucinous carcinomas. For a detailed description of mucinous carcinoma see the Invasive Mucinous Carcinoma page. |
 |
|
|
Carcinomas belonging to the category of neuroendocrine carcinoma vary in their morphological attributes. Those classified as small cell carcinoma look like the small cell carcinomas that arise in many other organs. Other varieties of carcinomas with endocrine features do not have specific names and are classified simply as invasive ductal carcinoma with endocrine features.
Small Cell Carcinoma
Small cell carcinoma originates in the breast so rarely that one must always exclude the possibility of a metastasis when confronted with a small cell carcinoma within the breast. One does so either by demonstrating an in-situ component or by excluding other primary sites by means of an exhaustive clinical evaluation. Like small cell carcinomas originating in other organs, mammary small cell carcinomas often stain for TTF-1, so immunohistochemical staining for this molecule will not aid in this distinction. Metastases from other rare tumors such as Merkel cell carcinoma can also mimic a primary mammary small cell carcinoma.
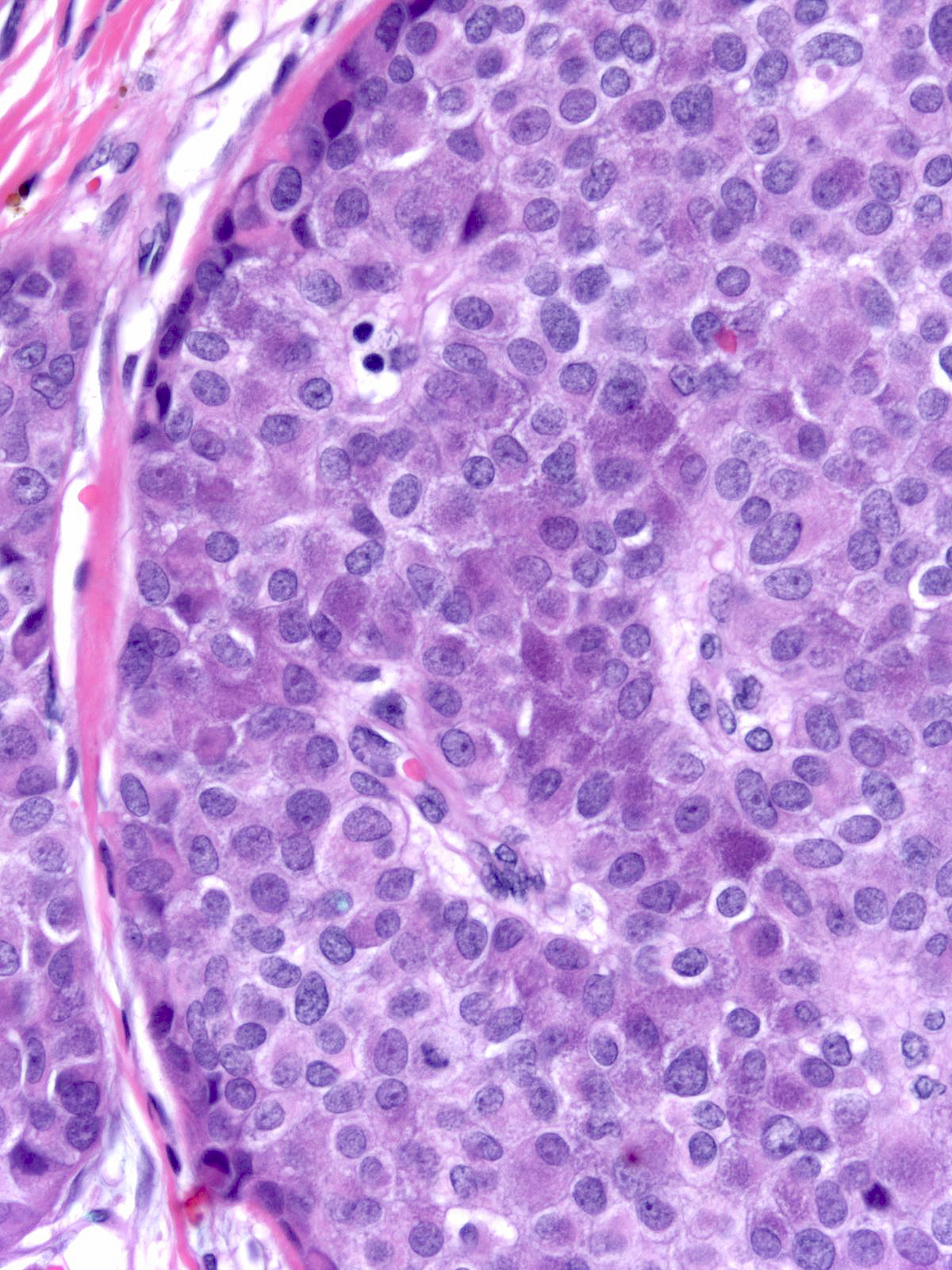
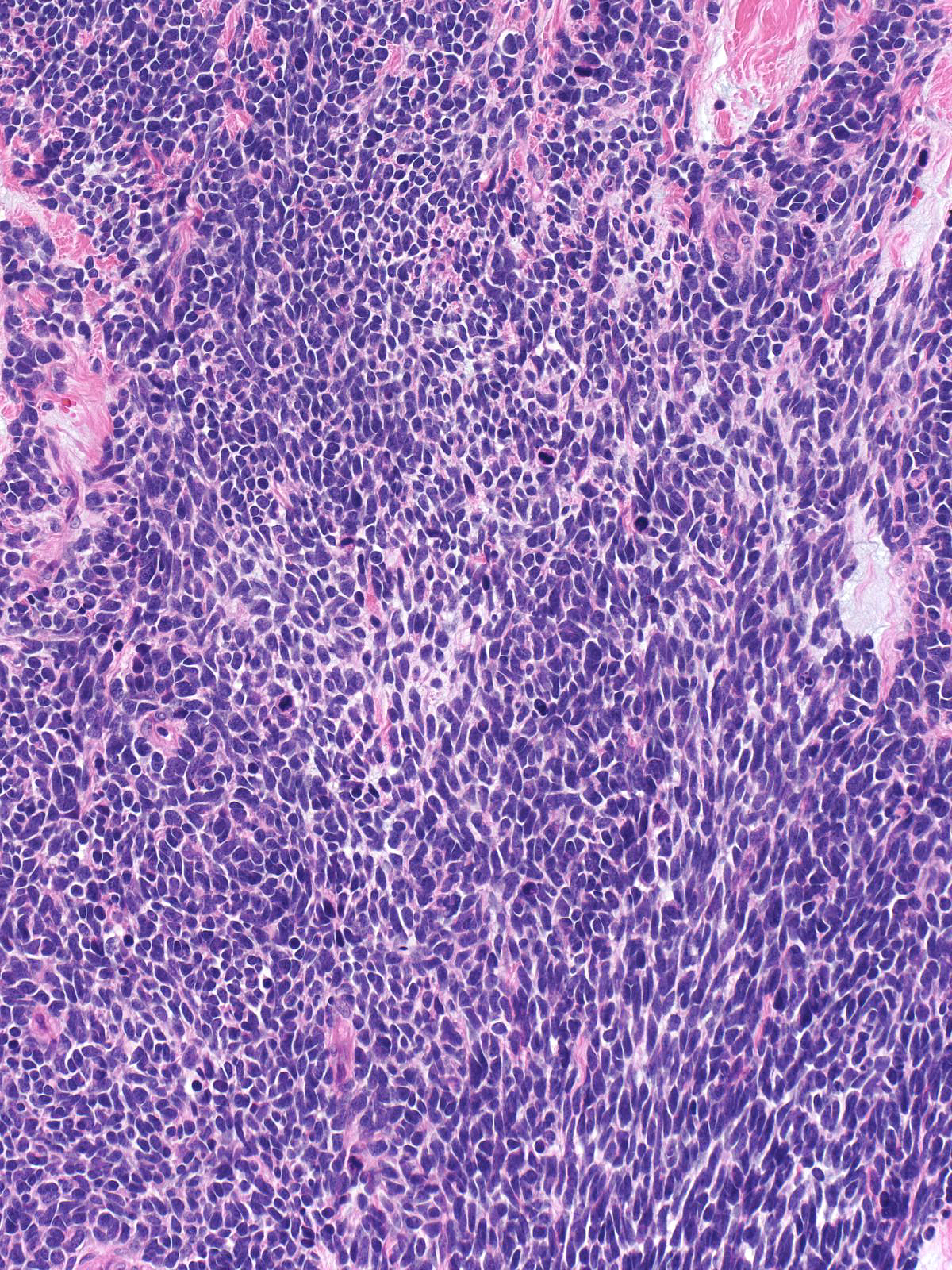
This carcinoma within the breast exhibits the typical morphological features of small cell carcinoma.
| The in-situ component seen here supports the notion that this small cell carcinoma is a breast primary. (In-situ and invasive small cell carcinoma) |
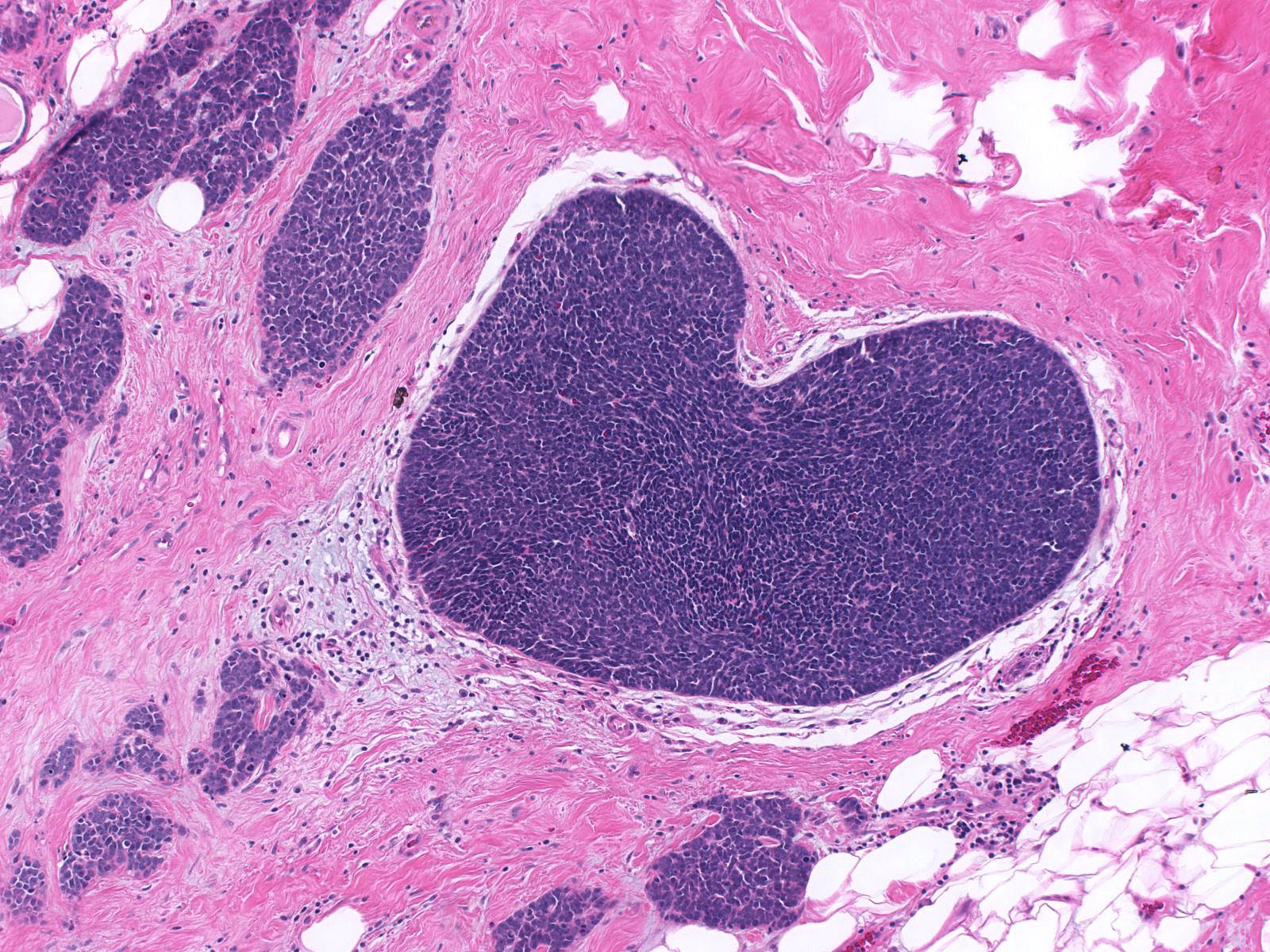 |
|
|
Other Carcinomas with Endocrine Differentiation
The morphological attributes of invasive ductal carcinomas with endocrine features vary depending on the grade and the growth pattern of the neoplastic cells, but the speckled chromatin characteristic of endocrine tumors represents a common thread.
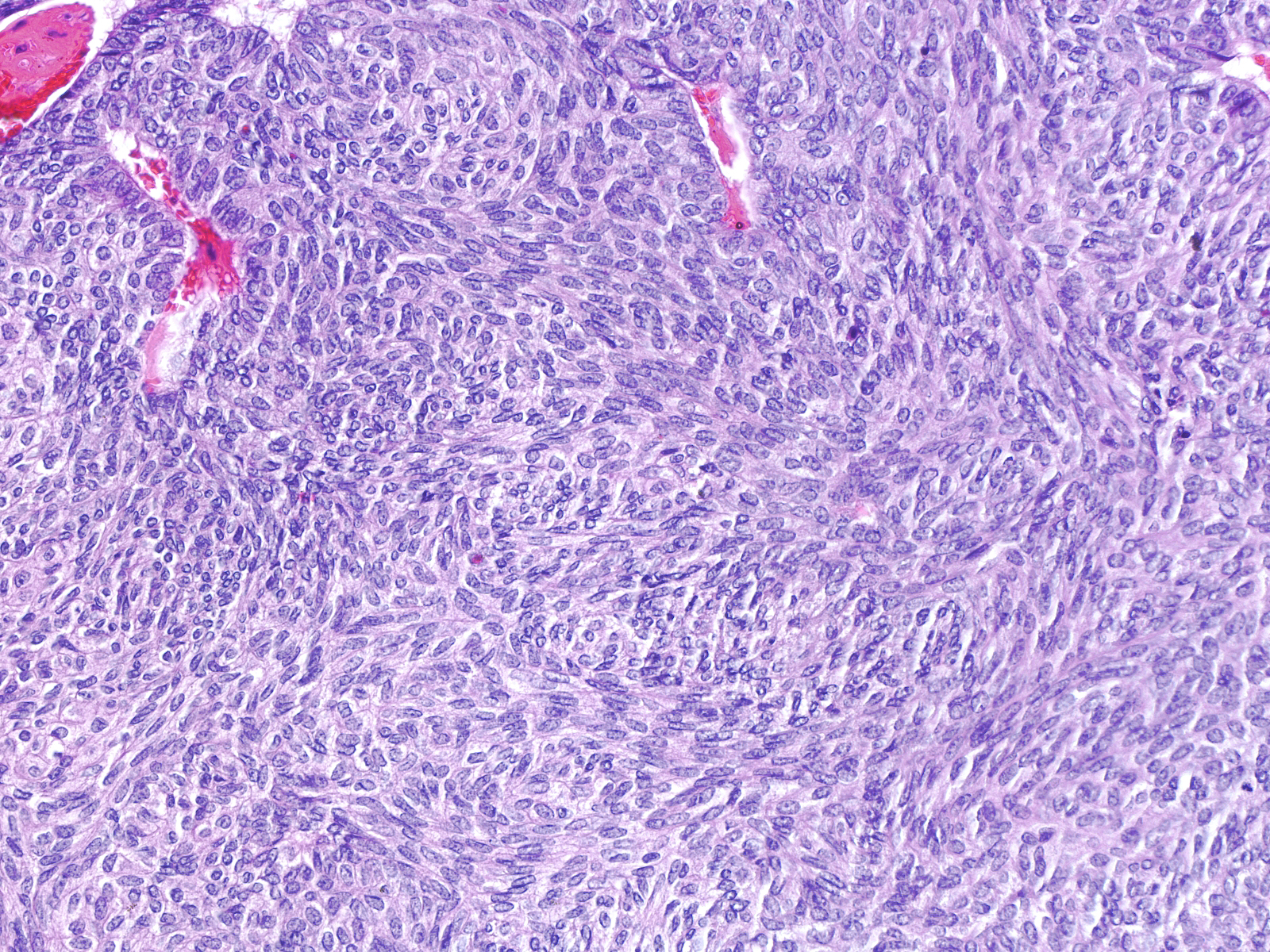
Low-grade invasive ductal carcinomas with endocrine features most commonly grow in a nested pattern, but trabecular and carcinoid-like arrangements occur rarely. The cells have round, oval, or spindly nuclei and modest amounts of eosinophilic cytoplasm.
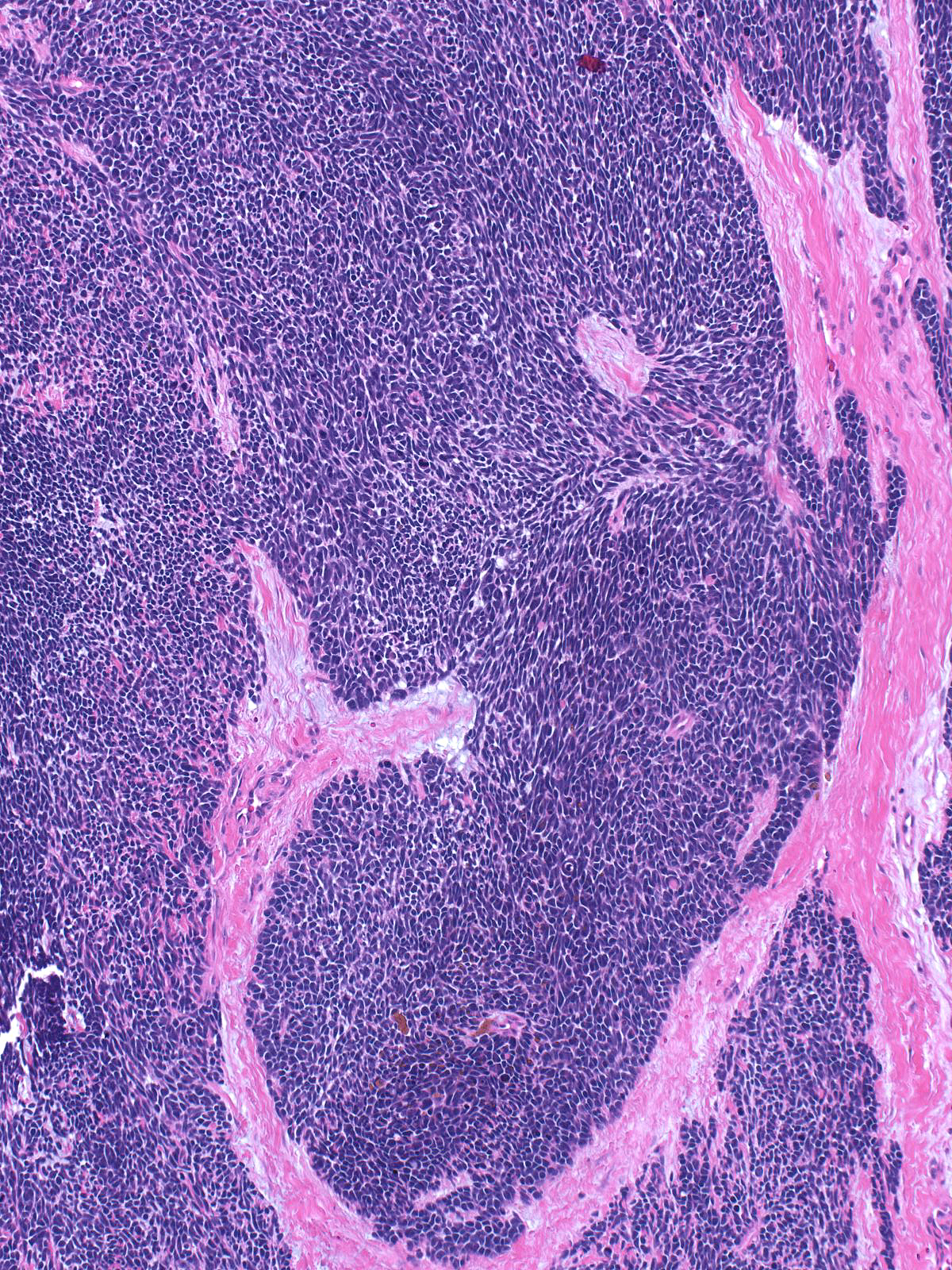
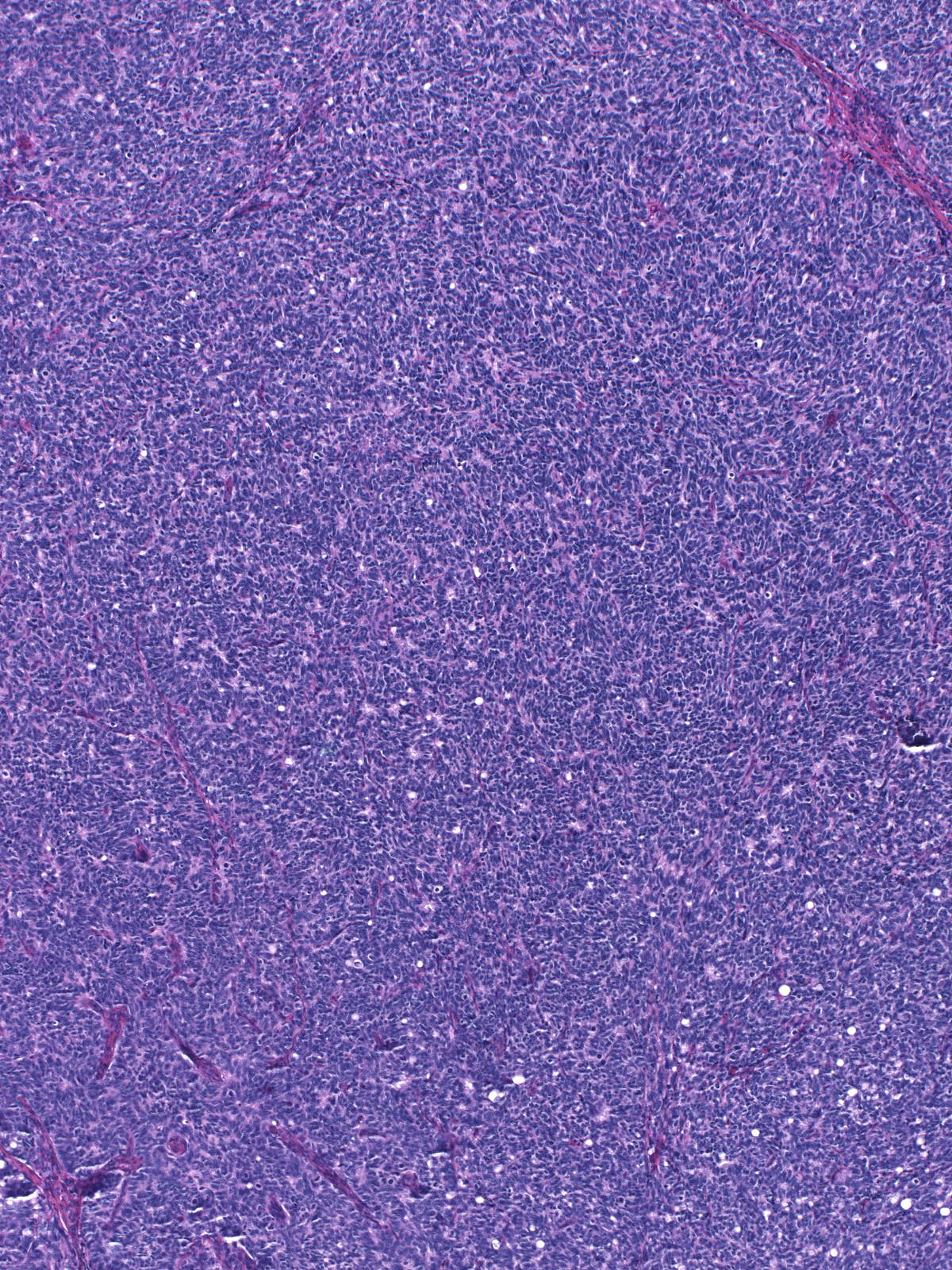
This low-grade invasive ductal carcinoma with endocrine features grows as confluent small clusters and aggregates. The cells have polygonal shapes and bland nuclei. They do not exhibit luminal characteristics.
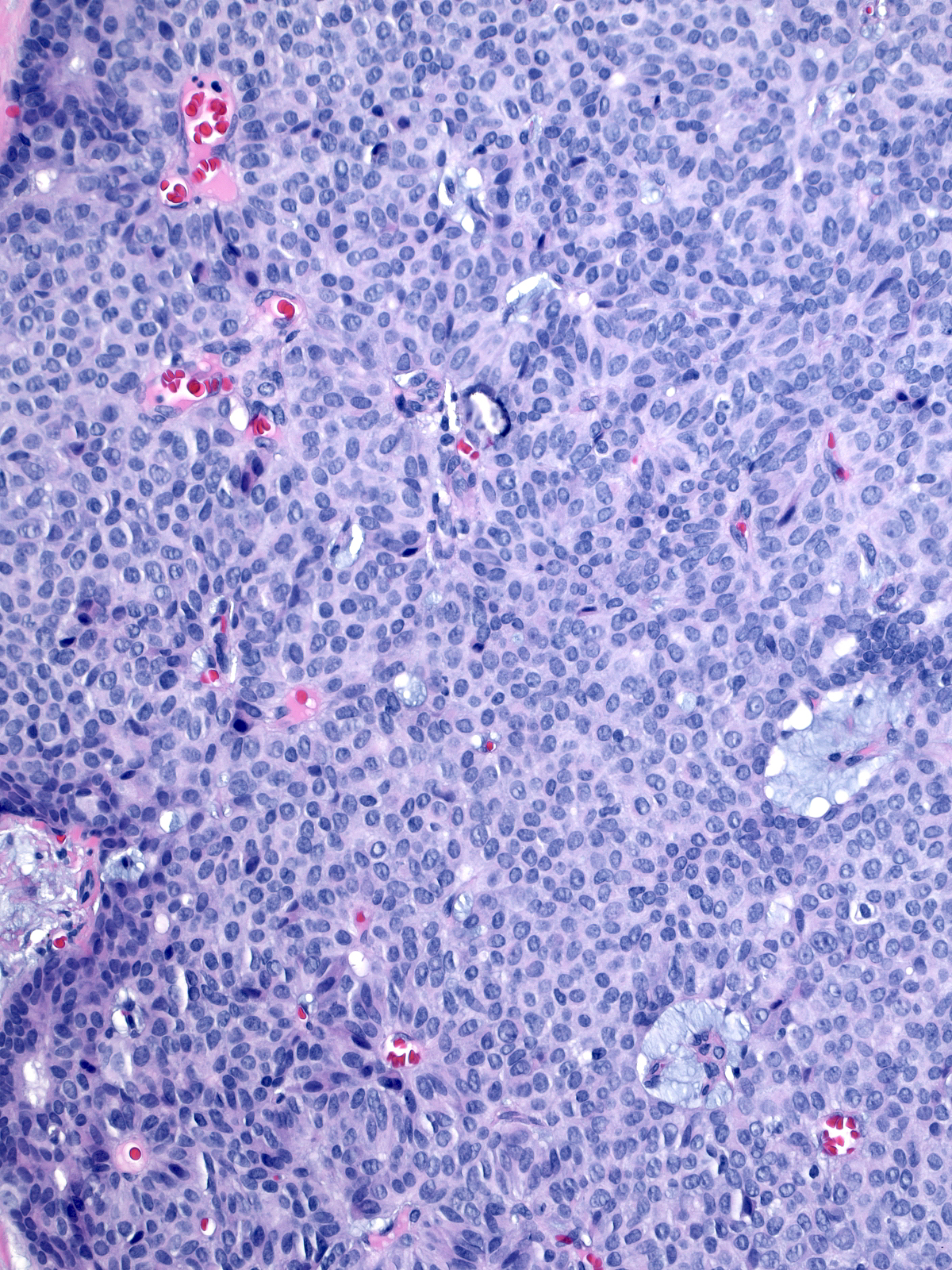
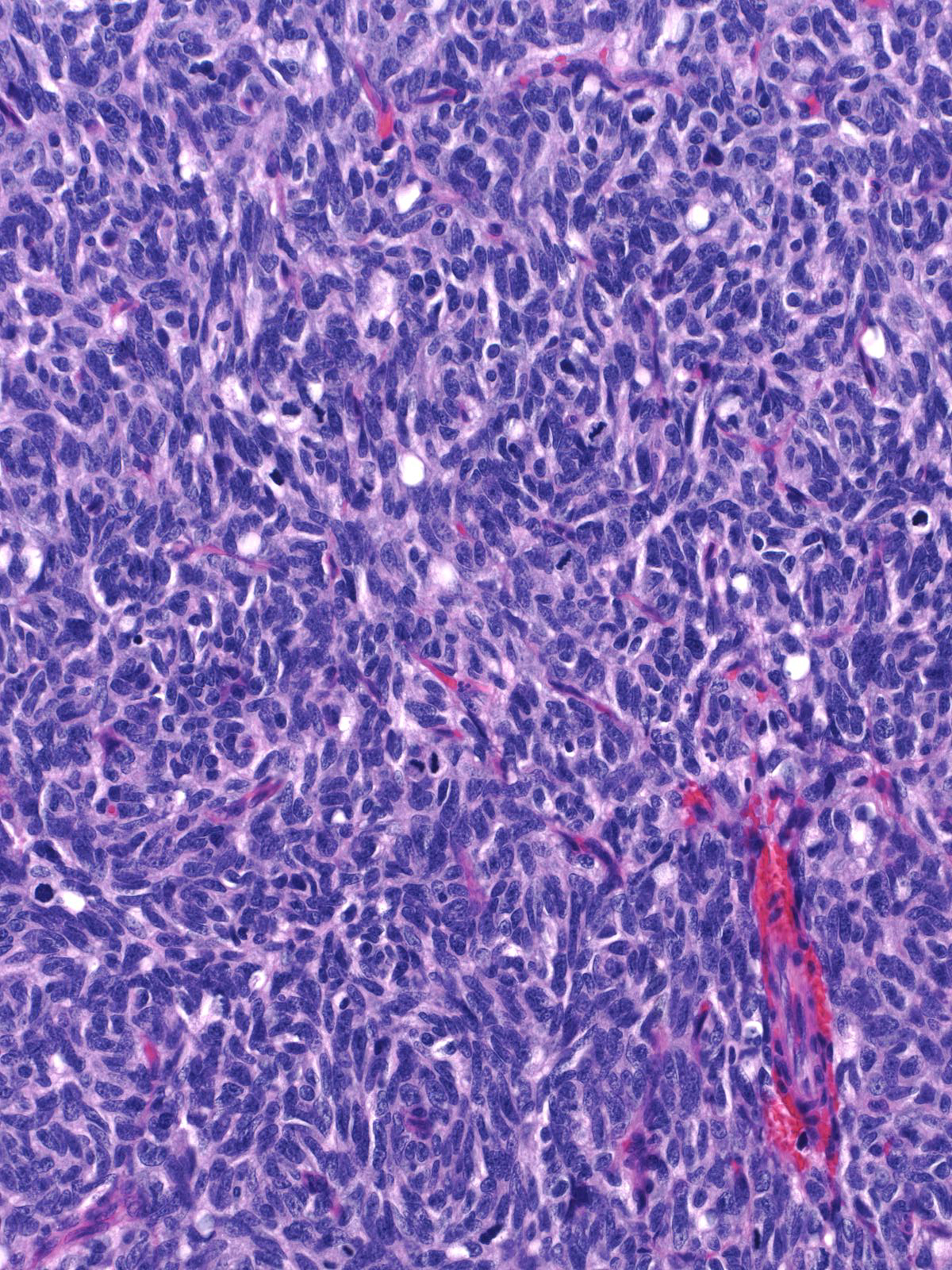
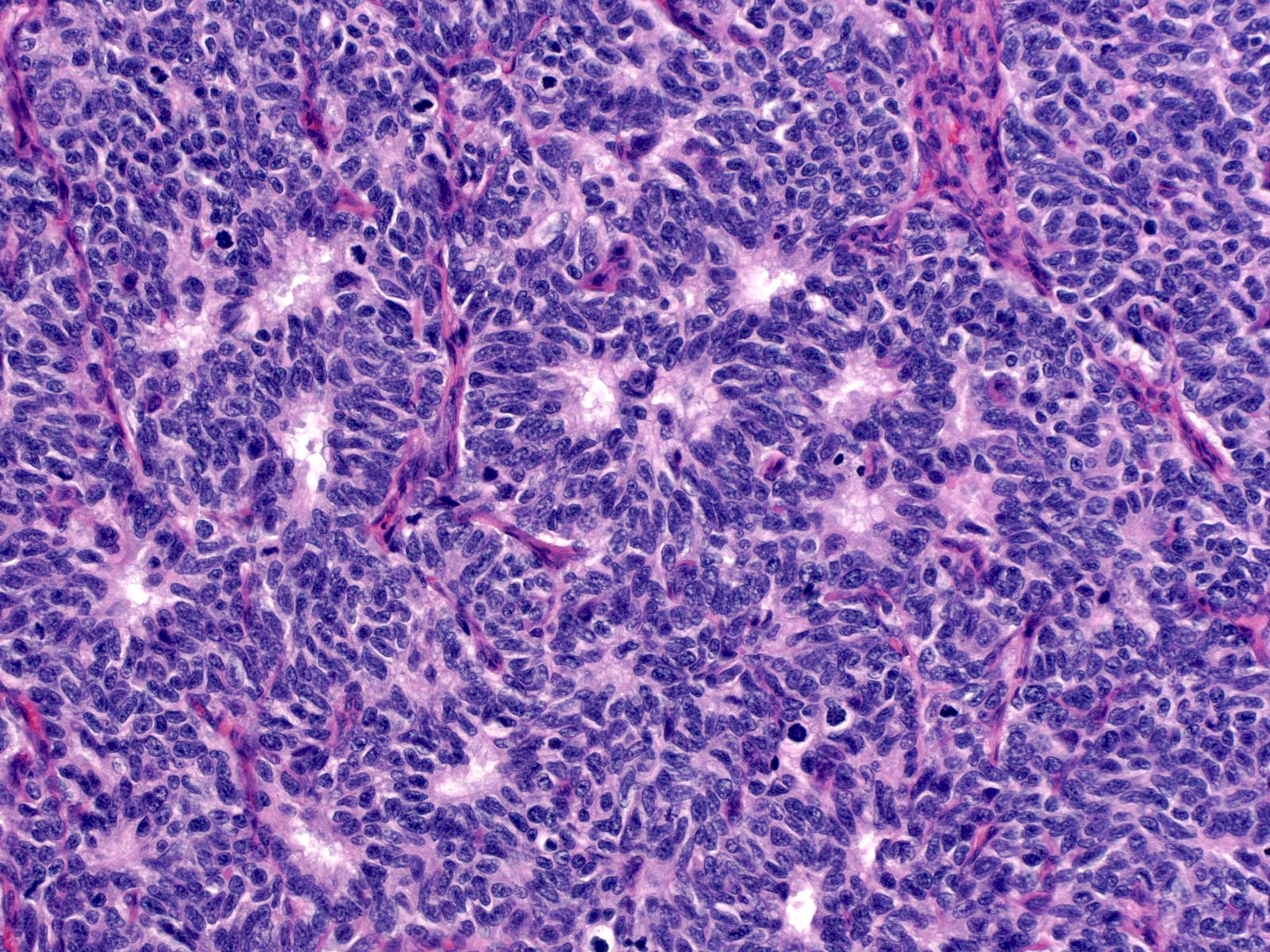
High-grade tumors grow in large masses composed of cells with oval to spindle shaped, dark nuclei and scant cytoplasm.
| One may observe nucleoli in high-grade tumors, and the cells can form rosettes. A capillary network sometimes permeates the tumor. |
 |
|
|
tumor) can demonstrate this finding.|24-7 Beta_cat.jpg}}
Breast Pathology