Difference between revisions of "mgh:cyto-1-8"
(Created page with "1-8 Thyroid Cytology: W. Faquin MD PhD, Lisa Ring CT Lecture slides: * [https://www.medialab.com/dv/dl.aspx?d=1324779&dh=4878c&u=111736&uh=c3cf8 Bethesda_System_for_Reporting...") |
|||
| (5 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | 1-8 Thyroid Cytology: W. Faquin MD PhD, Lisa Ring CT | + | __NOCACHE__{{DISPLAYTITLE:1-8 Thyroid Cytology: W. Faquin MD PhD, Lisa Ring CT}}{{:TOC}} |
| − | + | == Lecture slides: == | |
| − | Lecture slides: | ||
* [https://www.medialab.com/dv/dl.aspx?d=1324779&dh=4878c&u=111736&uh=c3cf8 Bethesda_System_for_Reporting_Thyroid_FNAs_and_Molecular_Testing.ppt] | * [https://www.medialab.com/dv/dl.aspx?d=1324779&dh=4878c&u=111736&uh=c3cf8 Bethesda_System_for_Reporting_Thyroid_FNAs_and_Molecular_Testing.ppt] | ||
<br> | <br> | ||
| Line 54: | Line 53: | ||
|}} | |}} | ||
<br> | <br> | ||
| − | + | == Basic cytomorphology == | |
| − | |||
<br> | <br> | ||
'''Benign Thyroid –THY4-16''' | '''Benign Thyroid –THY4-16''' | ||
| Line 64: | Line 62: | ||
* May find few macrophages (foam cells) | * May find few macrophages (foam cells) | ||
* Reactive Hurthle cell changes can be seen | * Reactive Hurthle cell changes can be seen | ||
| − | + | {{img4|1-8-1 watery_colloid.jpg|1-8-2 macrofollicle_benign.jpg|Watery colloid is usually a benign feature, with the exception of papillary thyroid carcinoma|Macrofollicles}} | |
| − | Watery colloid is usually a benign feature, with the exception of papillary thyroid carcinoma | + | {{img4|1-8-3 benign_follicular_lesion.jpg|1-8-4 ben+follicular+2.png|Single Endox Cells|Macrofollicles and colloid, consistent with a benign thyroid nodule}} |
| − | |||
| − | |||
| − | |||
| − | Macrofollicles | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | Macrofollicles and colloid, consistent with a benign thyroid nodule | ||
| − | |||
<br> | <br> | ||
| − | |||
'''Hashimoto Thyroiditis (chronic lymphocytic thyroiditis) –THY2-15''' | '''Hashimoto Thyroiditis (chronic lymphocytic thyroiditis) –THY2-15''' | ||
* 2 cell types NEEDED for diagnosis: lymphocytes and Hürthle cells! | * 2 cell types NEEDED for diagnosis: lymphocytes and Hürthle cells! | ||
| Line 89: | Line 73: | ||
* Tingible body macrophages and germinal center fragments | * Tingible body macrophages and germinal center fragments | ||
* Plasma cells | * Plasma cells | ||
| − | + | [[Image:1-8-5 hashimoto.jpg|thumb|Hurthle cells in Hashimoto thyroiditis are easily recognized as benign]] | |
| − | Hurthle cells in Hashimoto thyroiditis are easily recognized as benign | ||
| − | |||
<br> | <br> | ||
| − | |||
'''Papillary Carcinoma –THY2-26''' | '''Papillary Carcinoma –THY2-26''' | ||
* Increased cellularity! | * Increased cellularity! | ||
| Line 106: | Line 87: | ||
* SCANT colloid (or very little--dense, bubble-gum appearance) | * SCANT colloid (or very little--dense, bubble-gum appearance) | ||
* Psammoma bodies | * Psammoma bodies | ||
| − | + | {{img4|1-8-6 PTC.jpg|1-8-7 PTC_syncytial_groups.jpg|Papillary thyroid carcinoma (PTC) often results in a malignant or suspicious diagnosis|PTC with syncytial groups}} | |
| − | Papillary thyroid carcinoma (PTC) often | + | {{img4|1-8-8 PTC_longitudinal_nuclear_grooves.jpg|1-8-9 PTC+follicular+easy.png|PTC with longitudinal nuclear grooves|Easier to identify follicular variant of PTC}} |
| − | + | {{img4|1-8-10 PTC_follicular_harder.jpg|1-8-11 PTC+2.jpeg|Harder to identify follicular variant of PTC|PTC with abundant cytoplasm}} | |
| − | |||
| − | |||
| − | PTC with syncytial groups | ||
| − | |||
| − | |||
| − | |||
| − | PTC with longitudinal nuclear grooves | ||
| − | |||
| − | |||
| − | |||
| − | Easier to identify follicular variant of PTC | ||
| − | |||
| − | |||
| − | |||
| − | Harder to identify follicular variant of PTC | ||
| − | |||
| − | |||
| − | |||
| − | PTC with abundant cytoplasm | ||
| − | |||
<br> | <br> | ||
| − | |||
'''Medullary Carcinoma –THY4-05''' | '''Medullary Carcinoma –THY4-05''' | ||
* Malignant parafollicular C-cells | * Malignant parafollicular C-cells | ||
| Line 140: | Line 100: | ||
* Cell types include plasmacytoid, spindle, polygonal, round or triangular | * Cell types include plasmacytoid, spindle, polygonal, round or triangular | ||
* Amyloid--looks like dense colloid (stains bright green with Congo Red) | * Amyloid--looks like dense colloid (stains bright green with Congo Red) | ||
| − | + | [[Image:1-8-12 medullary+thyroid+carcinoma.png|thumb|Medullary thyroid carcinoma]] | |
| − | Medullary thyroid carcinoma | ||
| − | |||
<br> | <br> | ||
| − | |||
'''Undifferentiated (Anaplastic) Carcinoma –THY5-19''' | '''Undifferentiated (Anaplastic) Carcinoma –THY5-19''' | ||
* Increased cellularity | * Increased cellularity | ||
| Line 157: | Line 114: | ||
* May see mitoses | * May see mitoses | ||
* Background is usually rich in necrotic debris and blood | * Background is usually rich in necrotic debris and blood | ||
| + | {{:mgh:cytology-footer}} | ||
Latest revision as of 10:52, July 6, 2020
Contents
Lecture slides:
by William Faquin, M.D., Ph.D.
Thyroid nodules are discovered either by palpation or by an imaging study. A palpable thyroid nodule should undergo further evaluation to determine if an FNA is warranted. Before the decision is made, a serum thyrotropin level (TSH) and thyroid ultrasound (US) should be obtained. Patients with a normal or elevated serum TSH level should proceed to a thyroid US to determine if an FNA needs to be performed. Those with a depressed serum TSH should have a radionuclide thyroid scan, the results of which should be correlated with the sonographic findings. Functioning thyroid nodules in the absence of significant clinical findings do not require an FNA because the incidence of malignancy is exceedingly low. A nodule that appears either iso- or hypo-functioning on radionuclide scan should be considered for FNA based on the US findings.
Incidental thyroid nodules (“incidentalomas”) are detected by US, FDG-PET, sestamibi, CT, and MRI scans. Those detected by US have a cancer risk of approximately 10-15% (0-29%) and should undergo dedicated thyroid sonographic evaluation. Lesions with a maximum diameter greater than 1.0-1.5 cm should be considered for biopsy unless they are simple cysts or septated cysts with no solid elements. FNA may also occasionally be replaced by periodic follow-up for nodules of borderline size (between 1.0-1.5cm in maximum diameter) if they have sonographic features that are strongly associated with benign cytology.
A nodule of any size with sonographically suspicious features should also be considered for FNA. Sonographically suspicious features include microcalcifications, hypoechoic solid nodules, irregular/lobulated margins, intra-nodular vascularity, and nodal metastases (or signs of extracapsular spread). This recommendation is controversial because it includes patients with microcarcinomas, in whom a survival benefit following an FNA diagnosis has not been documented. If a sonographically suspicious nodule is benign by FNA, the patient can be reassured, and subsequent follow-up can be less frequent. On the other hand, if the FNA reveals that the nodule is malignant, surgery is generally recommended. The natural history of papillary microcarcinomas, however, is not well understood. Most remain indolent, as implied by the 13% prevalence of papillary microcarcinomas in the United States at autopsy examination. A minority follow a more aggressive course; this subgroup might be identified by sonographic evidence of lateral cervical node metastases, tumor multifocality, extrathyroidal invasion, or cytopathologic features that suggest a high-grade malignancy.
by William Faquin, M.D., Ph.D.
The fine-needle aspiration biopsy (FNA) was introduced for the first time in the U.S.A. in the 1930s but was only during the 1950s in Sweden that it was widely appreciated as a diagnostic tool. Since then this method has spread worldwide because of its simplicity, safety and the possibility of repetition.
The incidence and mortality of thyroid cancer do not qualify it as an important public health problem but the number of surgical lobectomies done to establish or to exclude its presence makes it a disease of economic importance. In this setting FNA is regarded as the most accurate method for the selection of patients with thyroid nodules and a very cost-effective diagnostic test.
The FNA of a thyroid nodule is preferably carried out under sonographic guidance, although when easily palpable, the maneuver can be performed under manual guidance. All the nodules in a multinodular goiter should be aspirated because the risk of malignancy is the same in each nodule but usually they are selected by the sonographer based on their ultrasound (US) appearance. A hypoechoic solid pattern with irregular margins and the presence of intralesional calcium deposits are the most important clues for suspecting a malignant lesion. Another useful method of nodule selection is the evaluation of its Echo-Power Doppler pattern: if a nodule is vascularized the likelihood of malignancy is higher compared to poorly vascularized lesions. The aspiration is performed with thin needles (gauge from 27G to 20G) and it is important to note that the amount of cells does not depend on the caliber of the needle but on the sampling time. Therefore, thyroid lesions which are usually richly vascularized are better sampled using very thin needles (either 27 or 25G) rather than larger ones (23 to 20G). After applying superficial anesthesia, which may be carried out by spraying the skin with ethyl chloride or by injecting lidocaine into the subcutis, the operator holds the sonographic probe with one hand and performs the aspiration with the other by means of a syringe-holding pistol. A FNA may also be carried out by simply moving the needle, without any connection to a suction device (cytopuncture): in this case the material is extruded from the lesion by capillarity. The risk of complications is low (see below) even when the number of FNA passes is up to 5 for each nodule. The procedure can be repeated safely when the smear shows low cellularity at the on-site assessment and a reliable diagnosis cannot be rendered. When on-site assessment of specimen adequacy is available, 2 passes are usually sufficient. However, when on-site evaluation is not possible or when liquid-based cytology is chosen 3-5 passes might be required depending on the skill of the operator and on the characteristics of the lesion.
There is no agreement in the literature as to whether the pathologist should or should not perform the FNA on his own. If the pathologist performs the aspiration, an immediate evaluation of the sample adequacy is possible. Clinicians are generally more familiar with the clinical aspects of the case; however, the different non-diagnostic rates by clinicians suggest that experience is an essential requisite to obtain an adequate cytological sample. Regardless of the subspecialty of the operator, the procedure should be carried out with appropriate frequency (at least 100 F.N.A.B./ year) to maintain competency.
by William Faquin, M.D., Ph.D.
When possible, the salivary gland FNA should be performed by, or in collaboration with, a cytopathologist in order that a preliminary interpretation of the sample can be made before the procedure is completed. This allows for assessment of sample adequacy, but it also permits the triage of the sample for ancillary studies. For example, the needle rinsings from lymphoid lesions can be sent for flow cytometric evaluation for potential lymphoproliferative lesions, a cell block can be made for cases where histochemical and immunohistochemical studies will be needed, microbiologic cultures can be sent for lesions that appear to be inflammatory/infectious, and a sample can be placed into glutaraldehyde fixative for ultrastructural studies on selected challenging cases.
A combination of both alcohol-fixed and air-dried smears are essential to maximize the diagnostic evaluation of a salivary gland FNA sample. Because many salivary gland tumors contain a variable combination of cells and matrix material, Diff-Quik and Papanicoloau stains are complimentary in the evaluation of salivary gland aspirates Diff-Quik staining highlights the cytologic features and tinctorial properties of any matrix material that may be present. This is key in distinguishing certain common salivary gland tumors such as pleomorphic adenoma and adenoid cystic carcinoma (see Chapter 6) where the appearance of the matrix rather than the cells is the most important diagnostic feature (Fig. 2.4). Diff-Quik stained smears are also more useful than Papanicoloau-stained preparations for the evaluation of cytoplasmic vacuoles as in acinic cell carcinomas (see Chapter 8). In addition, for the rapid assessment of an FNA specimen, air-dried Diff-Quik preparations are much less time-consuming to prepare. Alcohol-fixation and Papanicoloau staining is useful for better visualizing the nuclear features of the cell including chromatin pattern, nuclear membrane irregulaties, nucleoli, and inclusions. In our opinion, a detailed evaluation of nuclear atypia is best achieved using Papanicoloau staining.
As an adjunct to standard smears, needle rinsings are important since they can be used to produce a thin-layer preparation, cytospin and/or cellblock. Thin-layer (TP) or cytospin preparations in our opinion should not be the sole method for evaluating salivary gland lesions, especially those containing matrix material. TPs do have the advantage of concentrating the cells onto a single slide and of removing excess obscuring blood. For cystic lesions where the cellular components are diluted within a large volume, TP is probably the best preparatory method to use. A cellblock can be prepared from the needle rinsings for those cases where histochemical (e.g. mucicarmine, PTAH) or immunohistochemical (e.g. S-100, cytokeratin) stains would be useful in the diagnostic evaluation.
by William Faquin, M.D., Ph.D.
Salivary gland cytopathology is a diagnostically challenging area in part because of the wide variety of neoplasms arising in the salivary glands and the overlapping cytomorphologic features of so many of these tumors. As they say, “forewarned is forearmed;” by being aware of certain specific problem areas in salivary gland cytology, one can more readily avoid diagnostic errors. Within the context of the algorithm presented in Chapter 3 and applied within subsequent chapters of this book, we will address specific problem areas in detail. Below is a list of some of the most common problem areas of salivary gland FNA where the cytologist should be particularly cautious. Standard reporting terminology is used including: Non-diagnostic, Negative for malignant cells, Atypical, Suspicious for malignancy, and Malignant.
Diagnostically Challenging Areas of Salivary Gland Cytology: Matrix-containing lesions: pleomorphic adenoma versus adenoid cystic carcinoma Basaloid neoplasms: basal cell adenoma and basal cell adenocarcinoma versus solid variant of adenoid cystic carcinoma Oncocytic lesions: Warthin tumor and oncocytoma versus acinic cell carcinoma Mucinous cysts: low-grade mucoepidermoid carcinoma versus mucocele High-grade carcinomas: high grade mucoepidermoid carcinoma versus salivary duct carcinoma and metastasis Lymphoid lesions: lymphoepithelial sialadenitis (LESA) vs lymphoma Clear cell tumors: epithelial-myoepithelial carcinoma vs myoepithelioma Spindle cell lesions: schwannoma vs myoepithelioma
Basic cytomorphology
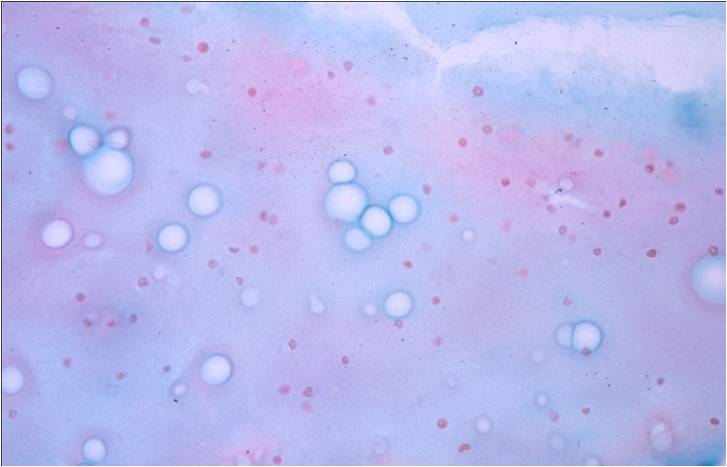
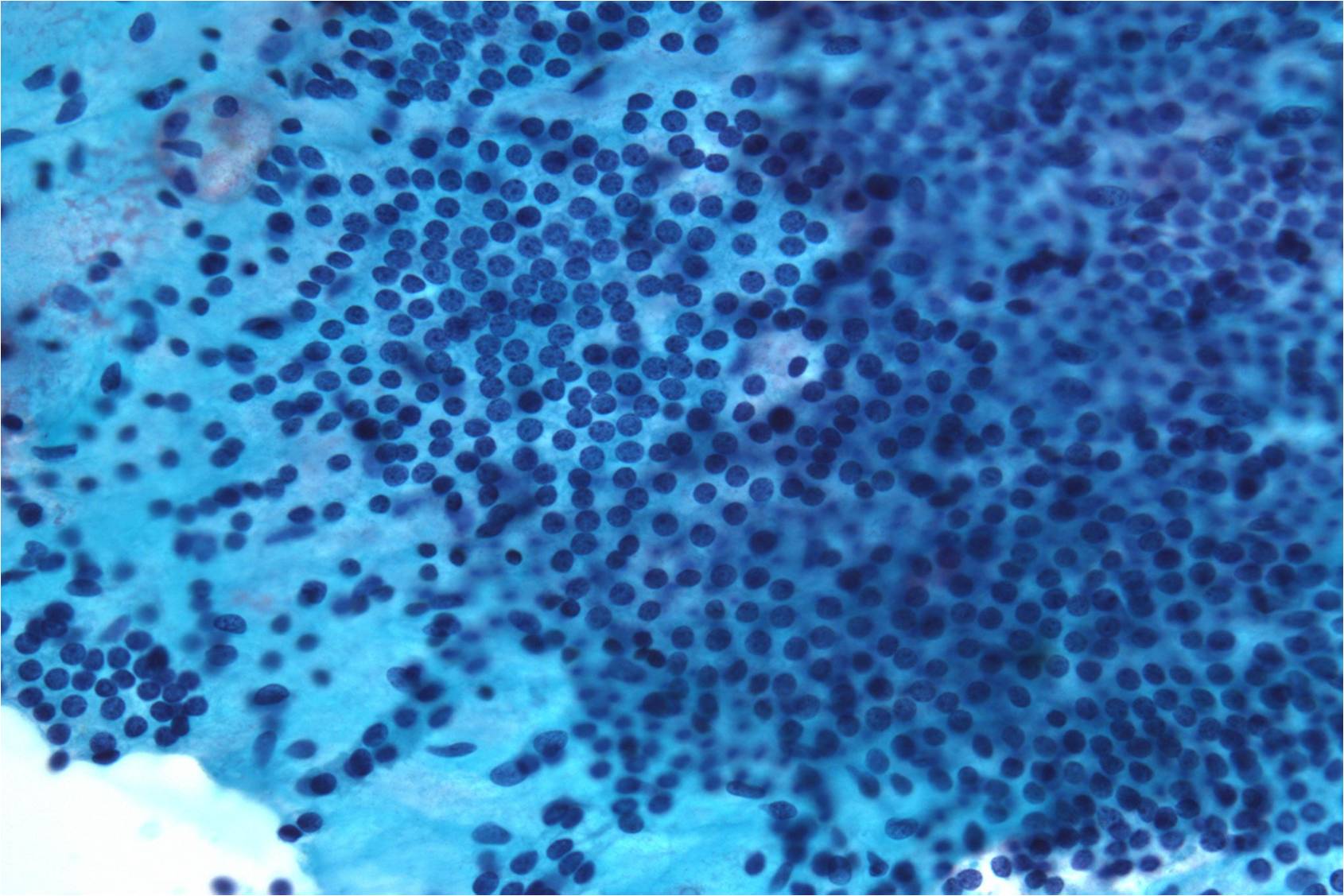
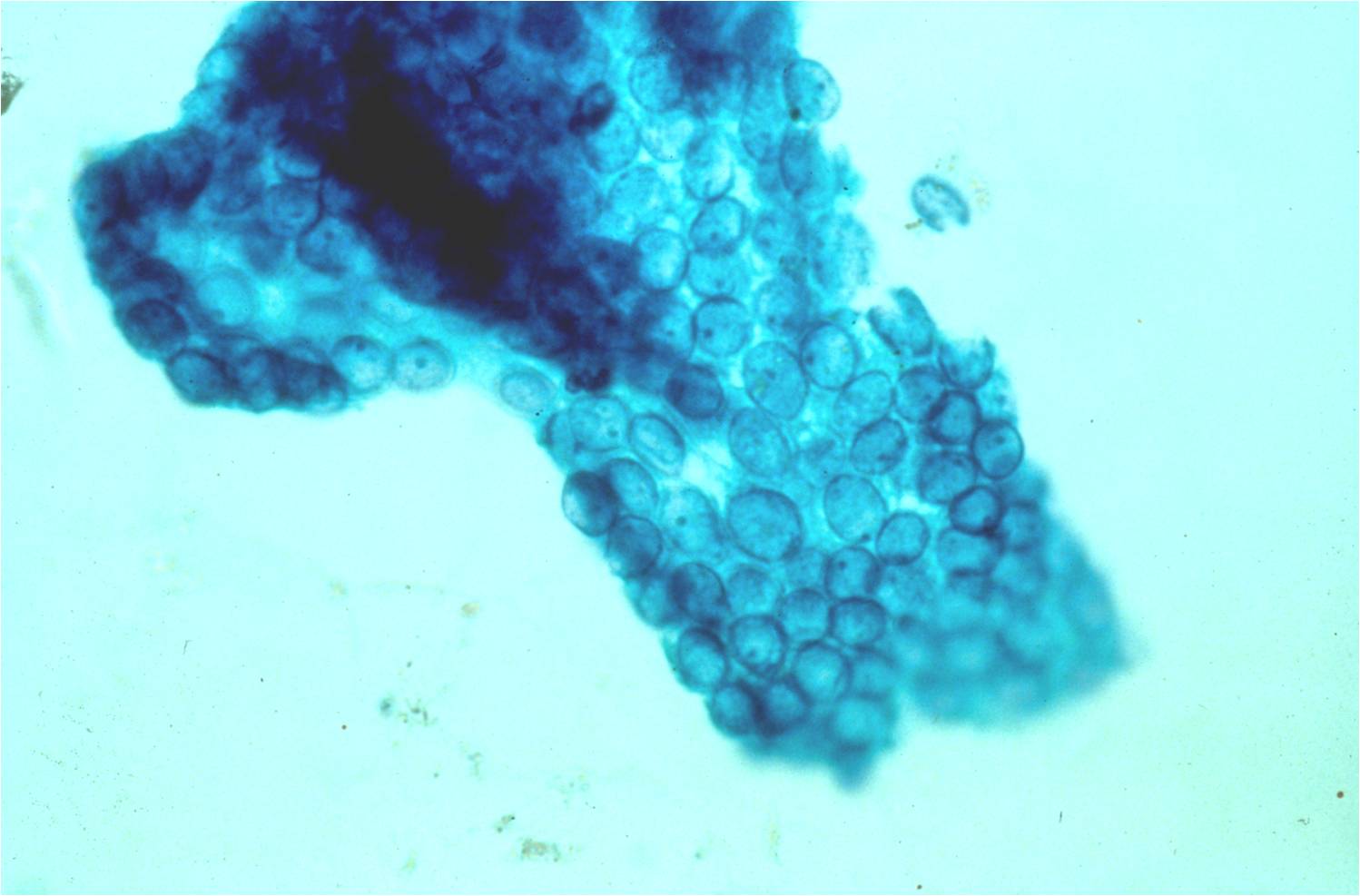
Benign Thyroid –THY4-16
- Adequacy: must meet a minimum of 6 groups of well-visualized follicular cells with at least 10 cells per group
- Abundant watery colloid in background
- Scattered fragments of macrofollicles
- Normal follicular cells contain small amounts of granular cytoplasm with a small, dark round central nucleus
- May find few macrophages (foam cells)
- Reactive Hurthle cell changes can be seen
 |
 |
 |
 |
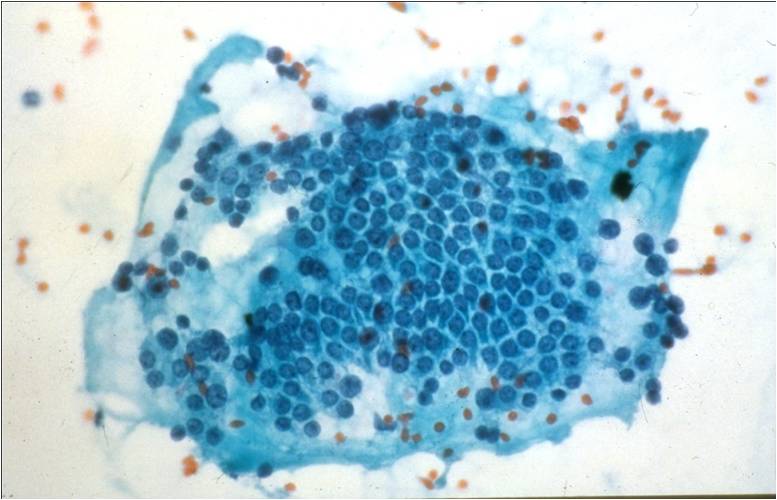
Hashimoto Thyroiditis (chronic lymphocytic thyroiditis) –THY2-15
- 2 cell types NEEDED for diagnosis: lymphocytes and Hürthle cells!
- Lymphocytes embedded within the groups of follicular/Hürthle cells are a characteristic feature
- Hürthle cells may show increased N/C ratio, prominent nucleoli and nuclear irregularity
- Small amounts of dense colloid may be present
- Squamous metaplastic cells, foam cells, giant cells, fibrous tissue, granulomas and calcifications may be present
- Tingible body macrophages and germinal center fragments
- Plasma cells
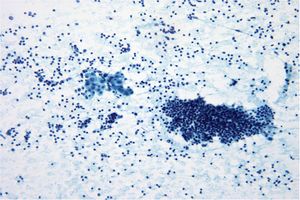
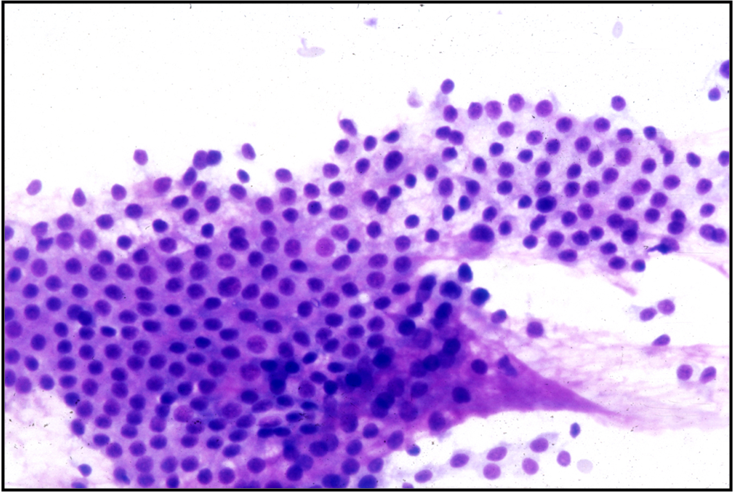
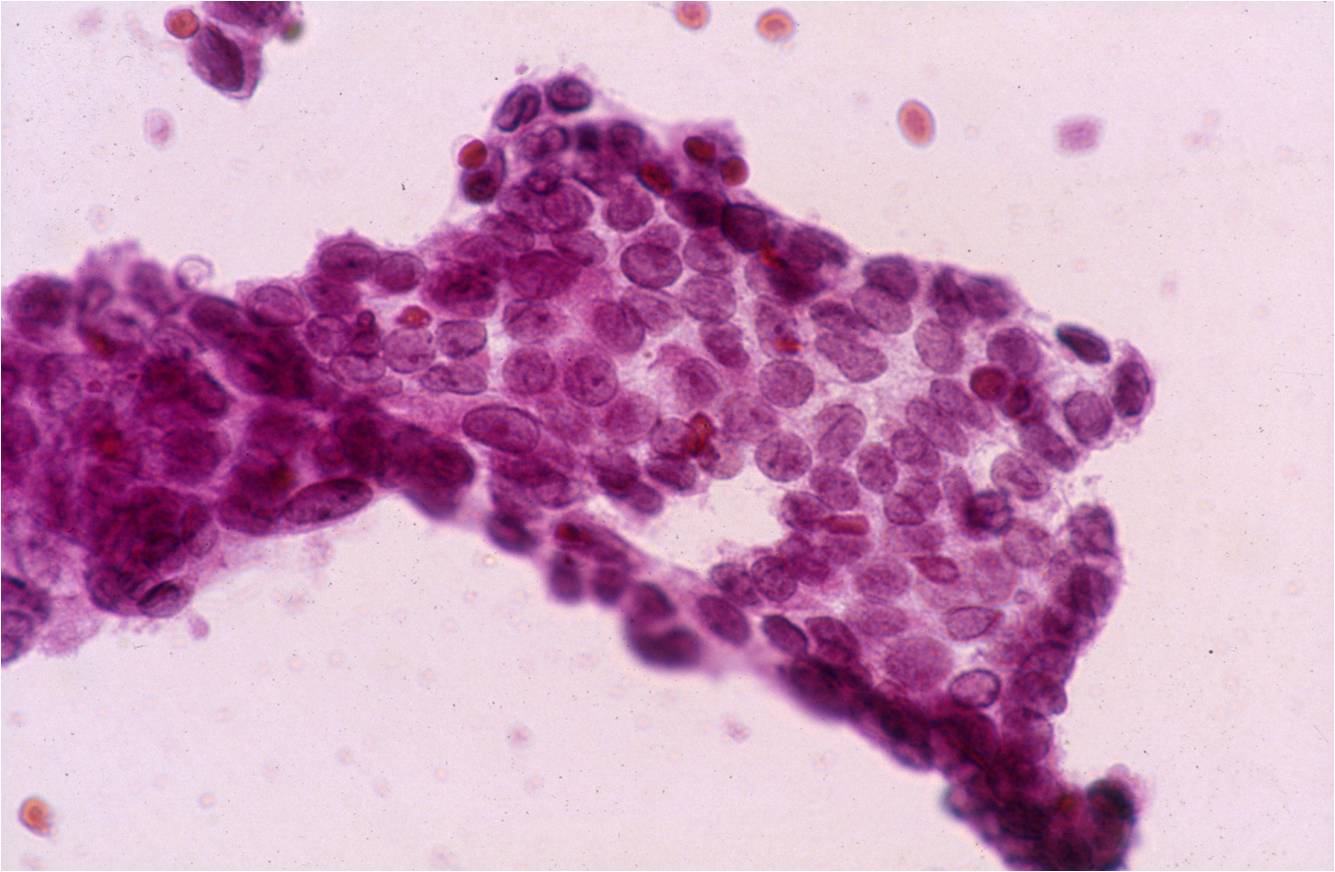
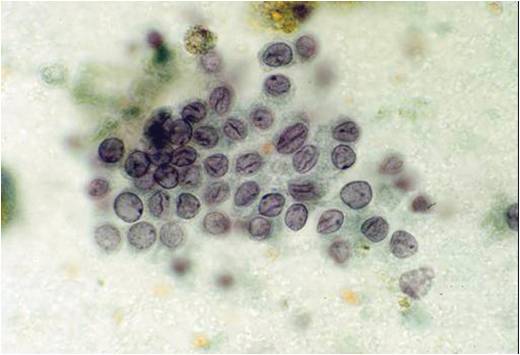
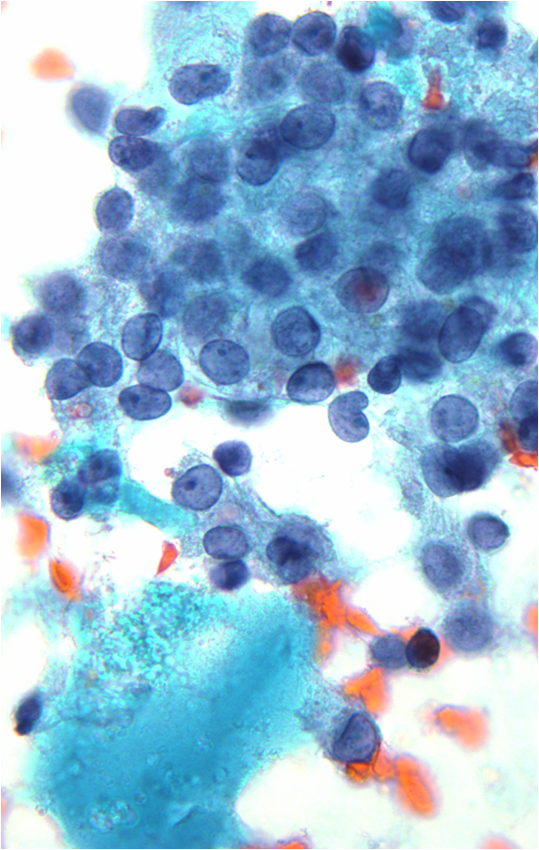
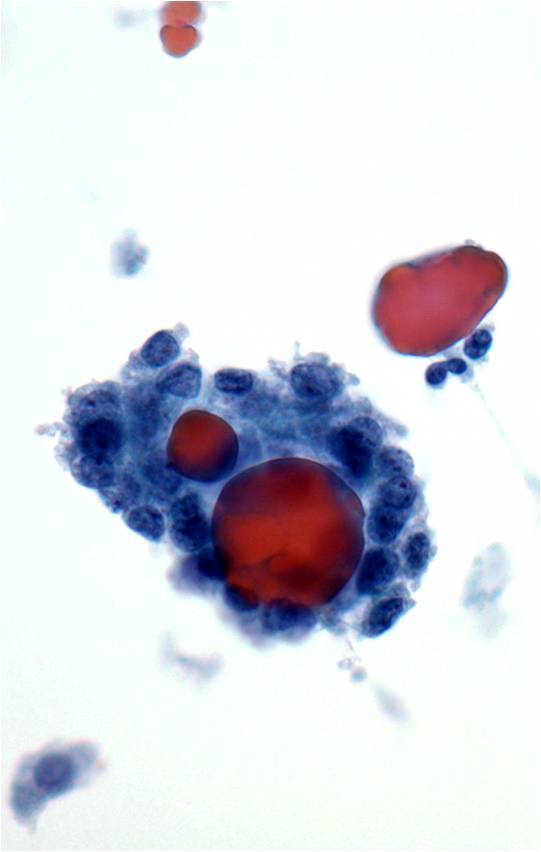
Papillary Carcinoma –THY2-26
- Increased cellularity!
- 3D papillary architecture or monolayered sheets
- Intranuclear inclusions
- Nuclear grooves (less specific for diagnosis)
- Pale even chromatin
- May see small but prominent nucleoli
- Variation in nuclear size and shapes
- Generally abundant cytoplasm that is either dense and homogenous or granular but well defined borders
- Blood in background
- SCANT colloid (or very little--dense, bubble-gum appearance)
- Psammoma bodies
 |
 |
 |
 |
 |
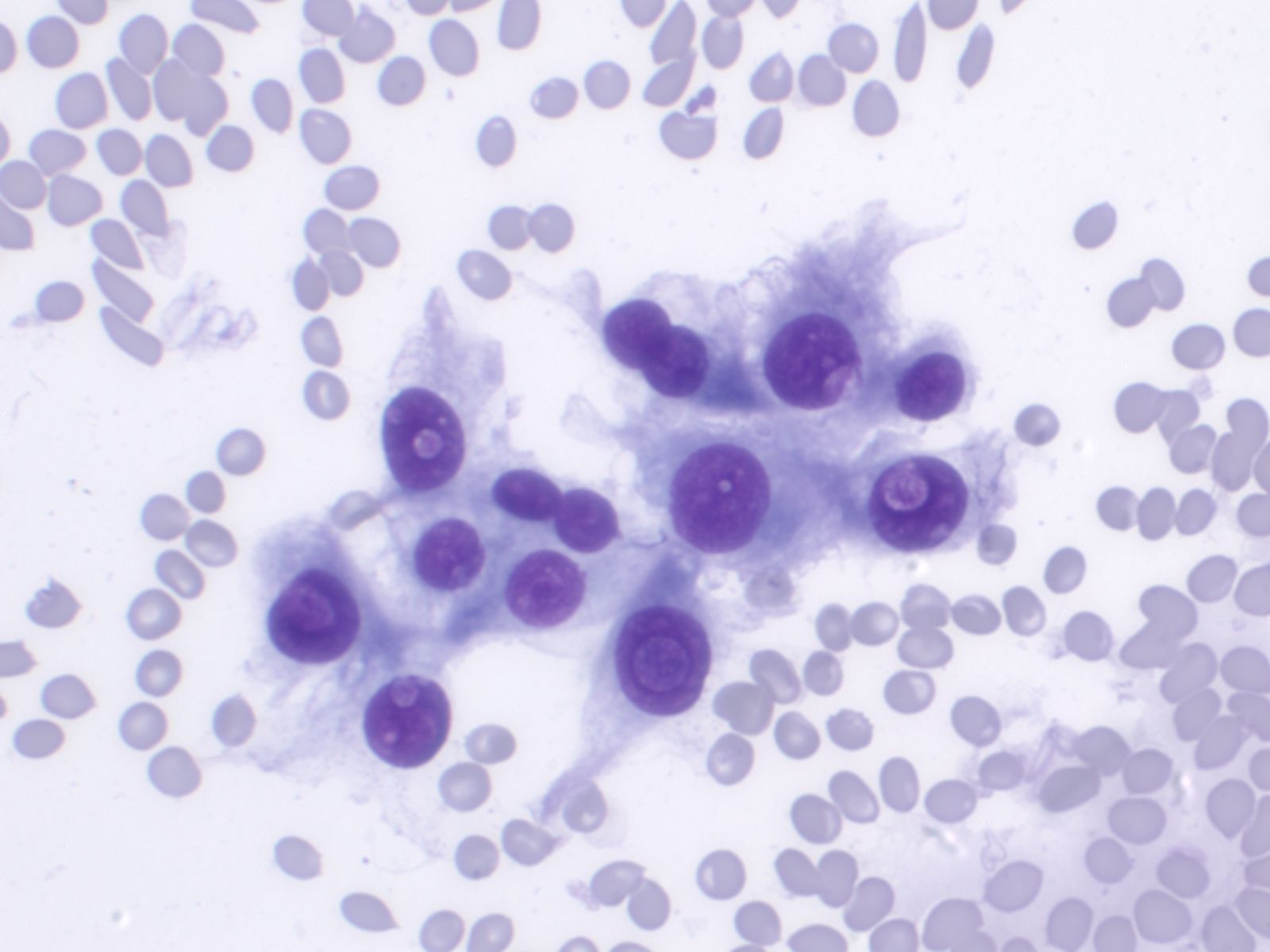 |
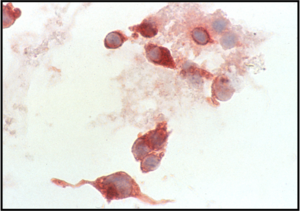
Medullary Carcinoma –THY4-05
- Malignant parafollicular C-cells
- CELLULAR SMEAR with isolated cells and some loosely cohesive cell clusters
- Mild to absent pleomorphism
- Can have a neuroendocrine-like appearance--salt & pepper chromatin pattern
- Intranuclear pseudoinclusions are also present
- Cytoplasm is finely granular and may contain red granules (seen in 30% of cases with Diff-Quik)
- Cell types include plasmacytoid, spindle, polygonal, round or triangular
- Amyloid--looks like dense colloid (stains bright green with Congo Red)
Undifferentiated (Anaplastic) Carcinoma –THY5-19
- Increased cellularity
- Malignant features
- Epithelioid cells present in groups and singly--round, spindled or polygonal in shape
- Pleomorphic nuclei--may see bi or multinucleated cells
- Increased N/C ratios
- Cytoplasm is moderate to abundant, basophilic and well-defined which sometimes may be vacuolated or granular
- Prominent nucleoli
- Irregular, clumped, coarse chromatin patterns
- May find intranuclear inclusions
- May see mitoses
- Background is usually rich in necrotic debris and blood